Group
Type of repair
No. of animals
1
SCEC saline
8
2
SCEC isogenic BMSC
8
3
SCEC allogenic BMSC
8
4
Autograft
8
Operative Technique
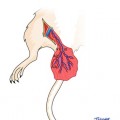
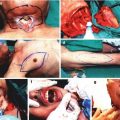
Anesthesia was induced using an intramuscular injection of a ketamine cocktail (Ketamine 30 mg/kg, xylazine 6 mg/kg, acepromazine 1 mg/kg). Aseptic technique was maintained by a single surgeon during microsurgical dissection under an operating microscope (Zeiss OP-MI 6-SD, Carl Zeiss, Goettingen Germany) for all surgical interventions. The right sciatic nerve was exposed using a gluteal muscle-splitting incision and dissected to expose the sciatic (Fig. 58.1). The nerve was transected proximally and distally to obtain a 2 cm defect. The sciatic nerve was then suspended on a straight irrigator (30 ga × 1″ (25 mm) and while suspended the nerve fascicles were removed with the aid of fine surgical forceps from both the proximal and distal ends (Fig. 58.2). This resulted in a 2 cm empty epineural tube conduit. Prior to stromal cell injection, any visible branches, as well as the distal opening, were dissected and closed using 10–0 nylon suture to mitigate leakage. Bone marrow stromal cells (BMSC) were stained with PKH-26 red dye and transferred to a syringe. They are then injected into the epineural sheath while ligating the proximal end and then transferred to a non-adherent flask filled with medium that is exchanged every 2–3 days for 10 days. This then creates a stromal cell epineural conduit (SCEC) (Fig. 58.3). After being in culture for 10 days, again a 2 cm sciatic nerve defect was created in the recipient and the SCEC was interposed using an epineural repair with four 10-0 interrupted nylon sutures at the proximal and distal ends (Fig. 58.4). After achieving adequate hemostasis, the wound was closed with 4-0 absorbable suture in a running fashion for the gluteal fashion and 4-0 absorbable suture for the skin. The left side was not operated on and used as an intraanimal control.
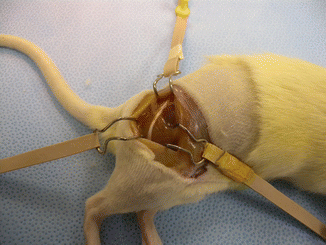
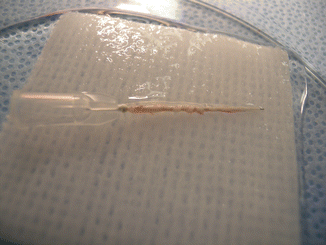
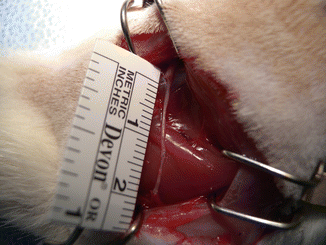

Fig. 58.1
Exposure of the sciatic nerve

Fig. 58.2
Sciatic nerve on a straight irrigator

Fig. 58.3
The SCEC in media

Fig. 58.4
The SCEC bridging the sciatic nerve defect
The autograft repair was done by taking a Lewis rat and performing the dissection described above and then a 2 cm defect was created on the right side. The nerve was then repaired using the piece of nerve that was cut out in order to create the defect. Four 10-0 nylon sutures were used to repair the proximal and distal ends.
Bone Marrow Stromal Cell Isolation
BMSC were obtained from either adult male Lewis or ACI rats. Fresh bone marrow was harvested aseptically from tibias and femurs. Both ends were cut and the marrow was flushed with 10 ml of alpha-MEM medium. After centrifugation the cell suspension was lysed with 0.85 % NH4 Cl. The cell suspension will then be filtered through 70 mm nylon mesh and resuspended with culture alpha-MEM medium supplemented with 10 % fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin, 100 ug/ml streptomycin and 25 ng/ml amphotericin B. The cells were then placed in a 75 cm2 flask and incubated at 37° celsius in 5 % CO2 for 3 days. At this time, the non-adherent cells were removed by replacing the medium. The culture medium was replaced three times a week. When the culture reached confluence, the cells were used for transplantation. The day of transplantation, the cells were stained with PKH red dyed prepared for injection into the epineural sheath.
Functional Analysis
Functional evaluation was performed by standard techniques established in our laboratory. Toe spread and pin-prick tests were evaluated at 3, 6, and 12 weeks. When a rat is held up by its tail, they will extend and abduct their hindfoot toes. The toe spread was graded between 0 and 3 by an experienced observer blinded to the group. Zero represented no movement, 1 signified any sign of movement, 2 was abduction, and 3 was abduction and extension.
A pin-prick was performed at the same time to evaluate sensory recovery. A pinching stimulus was applied to the limb from the toe to above the knee joint until a painful withdrawal response was elicited. Sensation was graded from 0 to 3, where 0 represented no sensation, 1 represented withdrawal above the knee, 2 and 3 represented a withdrawal response between the knee and ankle, and distal to the ankle respectively.
Electrophysiologic Assessment
At 12 weeks, electrophysiological assessment was performed by somatosensory evoked potentials (SSEP) using a Bio-logic A-PAX 486 computer (Biologica Systems Corp; Chicago, IL, USA). SSEP testing was conducted after anesthesia was induced using the above ketamine cocktail. Stimulating electrodes were placed subcutaneously in the operated limb and on the control side. Recording electrodes were placed within biparietal burr holes created using a handheld drill. Cortical SSEP were recorded from epidural electrodes with the active electrode placed over the contralateral midparietal cortex. The gain was set at 3 k, bandpass filter of 30–1,500 Hz, stimulation rate of 2.7 cycles per second, stimulus duration of 100 us, stimulus intensity of 4–6 mA, sweep of 10 ms/div.
Each average consisted of 300 trials and each average was replicated. Cortical potentials consisted of a series of negative and positive potentials. A characteristic waveform marked by an initial negativity (N1) followed by a positivity (P1) and a second negativity (N2) was consistently seen. P1 and N2 potentials were the most robust and consistent potentials. A percentage of the operated limb to the unoperated control limb was then calculated for each animal.
Muscle Denervation Atrophy and Evaluation
Muscle denervation atrophy was evaluated through comparative weights of the gastrocnemius muscle wet muscle mass. The gastrocnemius muscles, which are innervated by the posterior tibial nerve, were removed from both the experimental and nonoperated sides of each animal. The gastrocnemius muscle index (GMI) was calculated by dividing the weight of the muscle from the operative limb by the weight of the muscle of the unoperated control limb.
Statistical Methods
Ordered categorical data were summarized using frequencies and percentages, and continuous data were summarized using medians and quartiles. Kruskal-Wallis tests were used to evaluate overall relationships for the variables, while Wilcoxon rank sum tests were used for pair-wise comparisons. Analyses were performed using R software (version 2.8; Vienna Austria) and an overall significance level of 0.05 was assumed for all comparisons. No correction for multiple comparisons was performed.
Results
Macroscopic Observation
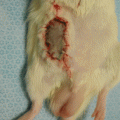
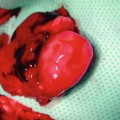
The nerve defect was bridged in all animals at the time of harvesting. There did not appear to be any signs of rejection. There was minimal fibrous tissue noted at the proximal anastomosis. We did not note any neuroma formation or axonal growth outside of the epineural tube (Fig. 58.5).
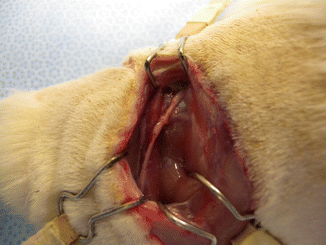

Fig. 58.5
The nerve defect was successfully bridged at the time of harvesting
Pin Prick Test
Two animals from the allogenic BMSC group, as well as two animals from the saline control group were assigned PP scores of 2. Otherwise, all other animals received a score of 3 with full sensory recovery. The differences were not statistically significant.
Toe Spread Test
The autograft repair had significantly better TS scores than the isogenic BMSC as well as the saline control groups (p = 0.003, p = 0.003). Toe spread scores were statistically comparable between the allogenic BMSC and the autograft repair.
Gastrocnemius Muscle Index
There was a significant statistical difference in comparing the autograft repair to all other groups. The GMI was significantly higher in the autograft group (0.42, Q1 = 0.36, Q3 = 0.50) compared to the allogenic (0.20, Q1 = 0.14, Q3 = 0.22) and isogenic (0.25 Q1 = 0.17, Q3 = 0.36) BMSC, as well as the saline control group (0.18 Q1 = 0.17, Q3 = 0.25) (p = 0.002, p = 0.007, p = 0.003).
Somatosensory Evoked Potentials
With regard to comparisons between P1 %, N2 % and Amp% values no significant differences were observed (p = 0.64, p = 0.21, p = 0.36). The saline control group trended towards a longer N2 latency (124 %, Q1 = 118 %, Q3 = 128 %) when compared to the allogenic (118 %, Q1 = 103 %, Q3 = 125 %) and isogenic (119 % Q1 = 110 %, Q3 = 133 %) BMSC groups, as well as the autograft (113 %, Q1 = 111 %, Q3 = 117 %). Interestingly, the saline control group did trend towards having the highest amplitude (61 %, Q1 = 34 %, Q3 = 84 %) compared to allogenic (34 %, Q1 = 20 %, Q3 = 41 %) and isogenic (47 %, Q1 = 30 %, Q3 = 64 %) BMSC and the autograft (44 %, Q1 = 39 %, Q3 = 71 %).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree