The Skin in Infective Endocarditis, Sepsis, Septic Shock, and Disseminated Intravascular Coagulation: Introduction
|
Infective Endocarditis
In the United States, the incidence of native-valve endocarditis is 1.7–6.2 cases per 100,000 person-years. The highest rate of infective endocarditis (IE) is seen in intravenous drug users, with an incidence estimated at 150–2,000 per 100,000 person-years. The risk of prosthetic valve IE decreases with time following valve implantation and the cumulative risk is about 2%–3% after 60 months.1
IE can be subdivided by native valve versus prosthetic valve. The causative organisms in most cases of IE are Staphylococci or Streptococci, implicating skin as the initial site of infection in many cases. Native valve endocarditis occurs most commonly in the setting of valvular disease or in people who use intravenous drugs. Rheumatic valve disease has been replaced by aortic stenosis and mitral regurgitation, as the most common settings in which native valve endocarditis is encountered. At sites of endothelial injury, a sterile thrombus can occur which is particularly susceptible to seeding of bacteria from transient episodes of bacteremia. This can result in the development of valvular vegetations that can cause local tissue destruction and embolic events.
Intravenous drug users are particularly susceptible to IE even though most have no preexisting structural heart disease. More than half of the cases of IE in injection drug users are right sided, involving the tricuspid valve. The most common causative organism is Staphylococcus aureus.
Prosthetic valve endocarditis can be classified as early (in the first 2 months following valve replacement) or late (2 months or later). The newly placed prosthetic valve is particularly susceptible to bacterial colonization because the process of endothelialization does not occur until 2–6 months following surgery. Early prosthetic valve endocarditis is most commonly caused by Staphylococcus epidermidis followed by S. aureus. Late prosthetic valve endocarditis can be due to a variety of organisms including the so-called HACEK organisms (Haemophilus parainfluenzae, Haemophilus aphrophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kinsella kinasae).
Most cases of nosocomial endocarditis are secondary to indwelling catheters or to surgical procedures, and are caused by Streptococci. However, nosocomial endocarditis in dialysis patients is most commonly due to S. aureus. Other less common causes of IE include fungi, Pseudomonas, Coxiella burnetii, Bartonella quintana, and Proprionobacteria acnes.
Patients with IE generally present with noncardiac complaints of fever, malaise, and anorexia. The presence of a murmur that is new or has changed in character is often present. Unexplained fever in a patient with a prosthetic heart valve should prompt an evaluation for endocarditis. The Duke criteria,2 (Box 181-1 are helpful in making the diagnosis of IE.
Major Criteria
Minor Criteria
|
Cutaneous signs of IE are nonspecific but may help the clinician in making the appropriate diagnosis. Cutaneous findings are caused either by embolic events, thrombosis, or focal vasculitis.
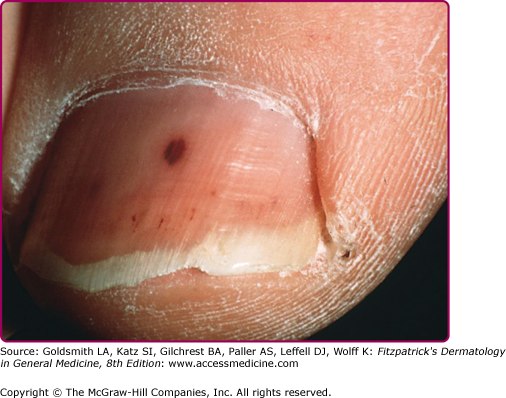
Splinter hemorrhages (Fig. 181-1) are 1–2 mm red–brown longitudinal streaks under the nail plate. They are seen in about 15% of patients with IE and are considered to be of greater diagnostic value if proximally located. Splinter hemorrhages associated with IE are the result of small capillary vasculitis, or from microemboli. In the absence of other signs or symptoms of IE, the mere presence of splinter hemorrhage is not specific enough evidence to warrant a workup. Crops of petechiae are commonly seen in the buccal membrane, soft palate, and extremities.
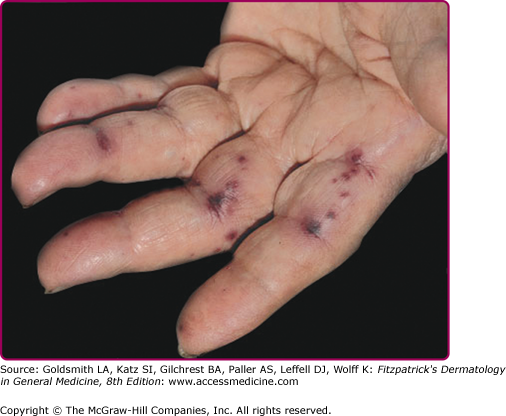
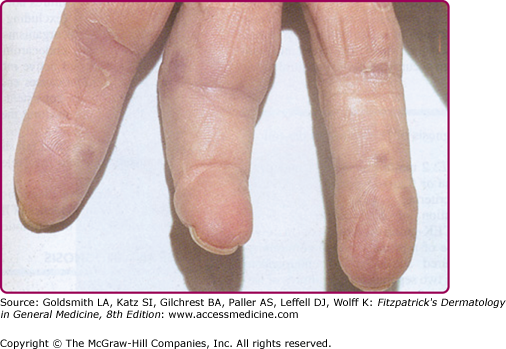
Janeway lesions are painless, irregular, nonblanchable, erythematous maculopapules that appear on the palms and soles and last days to weeks (eFig. 181-1.1). Histologically, thrombi are found in small vessels in the absence of vasculitis. Neutrophilic microabscesses can be seen in the dermis with occasional Gram-positive organisms. Osler’s nodes present as painful red papulonodules with a pale center on the fingertips lasting days to weeks (eFig. 181-1.2). Osler’s nodes are painful, perhaps due to involvement of the glomus bodies located in the fingertips. Histologically, neutrophilic microabscesses are present in the dermis, and arteriolar microemboli may contain Gram-positive cocci.
Septic emboli of valvular vegetation fragments can cause ischemia of distal extremities. Toes are most commonly involved, followed by fingers, and this condition presents as reticulated purpura similar to that seen in cholesterol emboli syndrome (see Chapter 174). While this condition generally resolves without sequelae, tissue necrosis or gangrene can result.
The most common noncutaneous finding in IE is fever. Signs and symptoms related to the bacterial infection and septic emboli may include splenomegaly, microscopic hematuria, pneumonitis, cerebral vascular accident, meningoencephalitis, brain abscess, retinal hemorrhages (Roth spots), acute abdomen, myocardial infarction, pericarditis and congestive heart failure, arthralgias and arthritis, or chronic wasting disease.
A positive blood culture is the most helpful laboratory test in making the diagnosis of IE. Other tests that may be abnormal in this setting include a positive rheumatoid factor, elevated erythrocyte sedimentation rate, C reactive protein, leukocytosis, or a urinalysis showing hematuria. Subacute bacterial endocarditis can be associated with the presence of antineutrophilic cytoplasmic antibodies (ANCAs).
Echocardiography allows the visualization of the valves and can provide information as to the level of myocardial involvement. Transthoracic echocardiography (TTE) is less invasive and less expensive than transesophageal echocardiography (TEE). However, the sensitivity and specificity of TTE are 60%–70% and 98%, respectively, whereas the sensitivity of TEE is 75%–95% without a compromise in specificity.
The differential diagnosis of splinter hemorrhages is reviewed in Box 181-2. Janeway lesions and Osler nodes can be similar in appearance to septic emboli and neutrophilic eccrine hidradenitis. Cases of culture-negative, ANCA-positive subacute bacterial endocarditis, particularly in the presence of cutaneous manifestations such as purpura, may mimic ANCA-associated vasculitis.
|
Cardiac complications of IE include congestive heart failure, extension of disease to the myocardium and pericarditis. Embolic complications include stroke, mycotic intracranial aneurisms and splenic abscess.1
The overall mortality for IE has dropped by about 50% over the past 40 years and is currently about 28%.2 Prognosis depends on age, the nature of the responsible microorganism, the presence of emboli, and the extent of cardiac damage. Early diagnosis and treatment improve outcome.
Appropriate antibiotic therapy should be initiated in suspected cases of IE after blood cultures have been drawn. Long-term (4–6 weeks) parenteral antibiotics, usually with a penicillin derivative, are used to treat streptococcal or staphylococcal IE. Antibiotic treatment has been shown to reduce the risk of subsequent embolism. Treatment of infective endocarditis caused by other organisms is reviewed elsewhere.5 Indications for surgical treatment include persistent bacteremia, despite 7 days of parenteral antibiotic therapy, prosthetic valve endocarditis, the presence of large vegetations, severe valvular dysfunction, and infection with Pseudomonas, C. burnetii, or fungus.6
Currently, prophylactic antibiotics for skin surgery are not indicated for procedures performed on noninfected, surgically scrubbed skin regardless of cardiac history. Guidelines issued by the American Heart Association in May 2007 shifted from recommending antibiotic prophylaxis for patients at increased risk for IE, to recommending IE prophylaxis for patient who have a high risk of an adverse outcome associated with IE who are to have a procedure that involves a contaminated or infected wound, or surgery on oral or nasal mucosa.7 For dermatologists, this would include patients with a prosthetic heart valve, a personal history of IE, valvulopathy in a cardiac transplant patient, or an unrepaired cyanotic heart defect.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree