Benign Neoplasias and Hyperplasias of Melanocytes: Introduction
Benign melanocytic proliferative lesions form a spectrum of disorders ranging from a massive accumulation of cells in multiple tissue elements to epidermal foci with a simple increase in epidermal melanocyte number. Here these lesions are separated into melanocytic neoplasias and melanocytic hyperplasias. The term melanocytic neoplasia is used to describe the presence of melanocytic cells in epidermal nests [defined as three or more melanocytic cells in direct contact (also known as thèque)], within the dermis, or in other tissues. The melanocytic neoplasms are referred to as nevi (singular: nevus) and the melanocytic cells forming these nevi are referred to as nevomelanocytes. The term melanocytic hyperplasia is used to indicate increased melanocytes confined to the basal layer of the epidermis.
The specific molecular events causing melanocytic neoplasias and hyperplasias are beginning to be defined. Given the presence of immature (less melanized) cells in many melanocytic neoplasias, it seems likely that specific underlying mutations (such as N-RAS, GNAQ, and B-RAF) disrupt normal melanocytic development and result in accumulation of the nevomelanocytic cells that do not complete typical migration and differentiation. The melanocytic hyperplasias are comprised of epidermal melanocytes at an increased concentration and thus alterations of normal melanocytic homeostatic mechanisms appear to be operative. These alterations could be due to a primary melanocytic defect [such as an ultraviolet (UV)-induced mutation] or homeostatic signaling changes in the local environment (possibly driven by mutations within the local keratinocytes, fibroblasts, or other resident cells).
This heterogeneous group of disorders is currently loosely subcategorized based on clinical features and microscopic characteristics. The neoplasias described include congenital nevomelanocytic nevi (CNNs), nevus spilus, common acquired nevomelanocytic nevi (excluding atypical/dysplastic nevi covered in Chapter 123), blue nevi, pigmented spindle cell nevi (PSCN), Spitz nevi, and nodal nevi. Cutaneous melanoma is covered separately in Chapter 124. The benign melanocytic hyperplasias described in this chapter include lentigo simplex (including agminated lentigines) and solar lentigines.
Congenital Nevomelanocytic Nevi
|
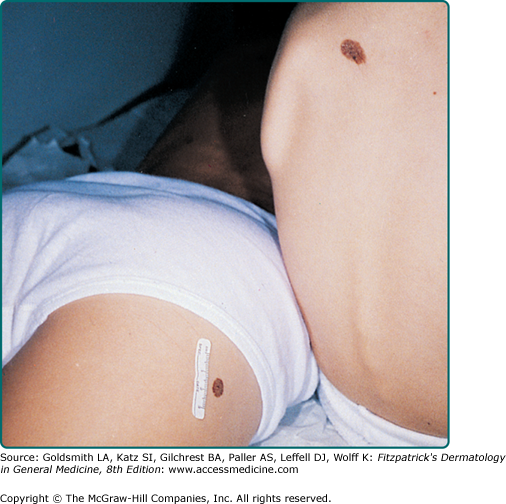
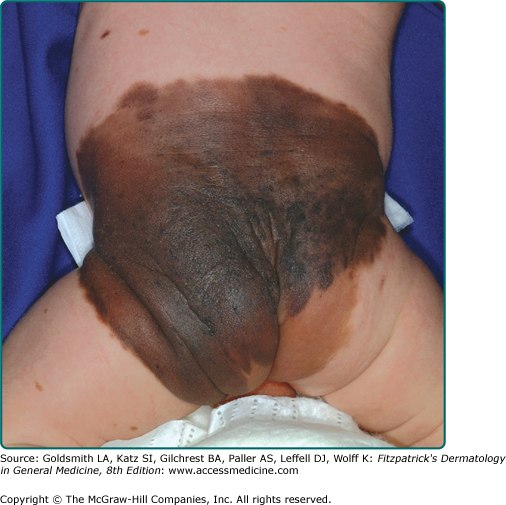
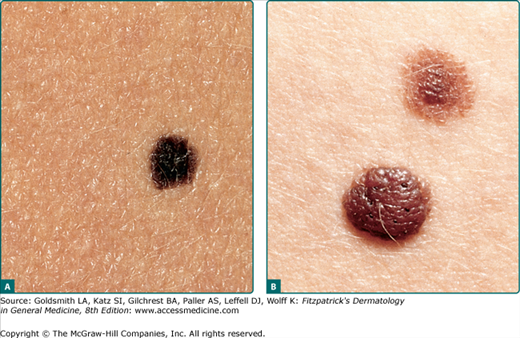
The vast majority of CNNs noted at birth are small and singular, and no gender predilection has been demonstrated. The most reliable prevalence rate for CNN in a homogeneous ethnic group was obtained by biopsying all pigmented lesions in 841 white infants examined within 72 hours of birth: of 21 infants with pigmented lesions, 7 babies (0.83% of 841) had a biopsy-confirmed nevomelanocytic nevus.1 According to other studies, the prevalence of CNN appears to be slightly higher in nonwhites compared to whites. Many other series have yielded equivalent results. CNNs of 99 mm or more in diameter occur in only 1 of every 20,000 newborns, and CNNs with a garment distribution occur in only 1 of every 500,000 newborns.2 Familial aggregation has been demonstrated for both large and small varieties of CNN (Fig. 122-1). Discordance for giant CNNs has been demonstrated for identical twins in at least three cases and for nonidentical twins in two cases.
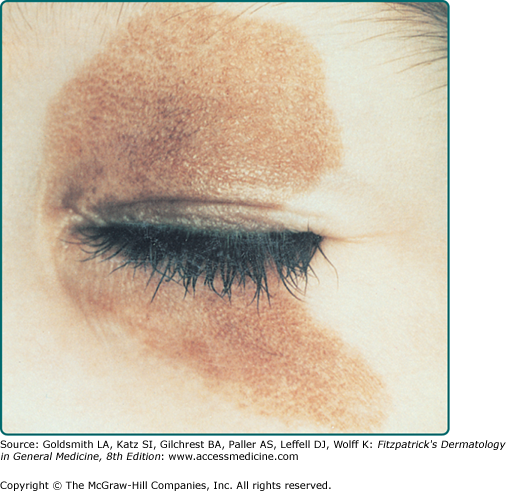
CNNs represent a developmental abnormality of normal melanocytic development. This is presumably due to a mutation (often NRAS)3 that occurs in a progenitor cell that results in the abnormal extensive accumulation of melanocytic cells along migration pathways during normal development. The events leading to the nevomelanocyte accumulation may also have effects on the surrounding tissue (i.e., increased length/darker hair) possibly due to changes in the local cytokine environment of the nevomelanocytic cells. Clinical findings such as a congenital divided nevus of the eyelid (Fig. 122-2) can give us insight into when these events occur.4 The eyelids form at between 5 and 6 weeks in utero, begin to fuse at about 8 to 9 weeks, and reopen during the sixth uterine month.5 Because of the contiguous nature of this lesion on the upper and lower eyelids, it may be presumed that the nevomelanocytes migrated into this location sometime during or after eyelid fusion but before the eyelids split.
Most CNNs are present at birth; however, there are also rare varieties of relatively large nevomelanocytic nevi (>1.5 cm) that appear for the first time between 1 month and 2 years of life, according to parental observations and corroborated by photographs (tardive congenital nevi). As a child grows, the CNN should grow relatively proportionally6 and continue to “mature.” History of disproportionate growth, especially after 6 months, or change in a nonuniform manner is of concern for possible melanoma. Both small and large varieties of CNNs have been associated with loss of pigmentation, halo depigmentation, and even regression.
Although CNNs are on average larger than acquired nevi, for lesions less than 1.5 cm in diameter there is no specific size limitation that can be used to predict reliably whether a given nevus is congenital or acquired. Lesions attaining a diameter of 1.5 cm or more are likely to be congenital, atypical melanocytic nevi, or melanoma.
There is no completely satisfactory way to categorize CNNs as small or large. Definitions have been based on ease of removal and termed small if they can be excised and the wound defect closed primarily without significant deformity. Arbitrary size criteria have also considered small (<1.5 cm; see eFig. 122-3.1), medium (1.5–19.9 cm), and large (≥20 cm). Size, of course, is all relative to the affected anatomic location and the patient. Giant has been variously defined as a lesion as large as the patient’s palm if it occurs on the head and neck (and twice that area for other anatomic sites), 30% of the body surface, or 900 cm2 in adults (or smaller if it involves a major anatomic area) (see Fig. 122-4). Depending on the definition used, lesions that are regarded as small or medium in the newborn period may be designated medium or large, respectively, by late childhood or adulthood, given that CNNs appear to grow in proportion to the affected anatomic site.6
The contour of CNNs is usually smooth, regular, and sharply demarcated, and skin markings distort the skin surface at least slightly when assessed by oblique lighting. Some CNNs are relatively hairless. However, coarse, long, darkly pigmented hair may be present at birth, appear within the first year or two of life, or be delayed for several years. Lesions may have a smooth, pebbly, rugose, verrucous, cerebriform, or grossly lobular surface. CNNs with a cerebriform appearance may present as cutis verticis gyrata.
Dermoscopy may reveal a reticular pattern or globular/cobblestone pattern and may be useful in the identification of small foci of melanoma. In large congenital nevi, there can be significant variability in pigmentation and structure, and melanomas may develop in the deep components potentially reducing the utility of dermoscopy in these lesions.
Unique varieties of CNNs may have an atypical appearance and be striking for their haphazard, very dark-brown, black, or blue–black pigmentation or discontinuous pigmentation and poorly demarcated and/or irregular outlines, often associated with atypical histopathologic features. Very darkly pigmented CNNs are distinctly uncommon in whites and should suggest the possibility of atypical histopathologic features. In darkly pigmented infants, CNNs are usually darkly pigmented.
CNNs of the head, neck, or posterior midline, and/or the presence of multiple satellite lesions associated with large CNN may be complicated by underlying cranial and/or spinal leptomeningeal melanocytosis. This phenomenon may be asymptomatic or may give rise to communicating or noncommunicating hydrocephalus, seizures, focal neurologic deficits, mental retardation, or even melanoma. CNNs need not be giant to be associated with this underlying disorder.
There is a significant association between neurofibromatosis and giant CNNs. In a study by Crowe et al,7 3 of 223 patients with neurofibromatosis had extensive CNNs. In his monograph on neurofibromatosis, von Recklinghausen described 1 of 28 patients as having a giant CNN.8 Tumors indistinguishable from neurofibroma in the absence of von Recklinghausen neurofibromatosis may develop in association with giant varieties of CNN.
Malignant degeneration of large CNNs may be associated with the relatively sudden appearance of a dermal or subcutaneous nodule, very dark pigmentation, itching, pain, bleeding, or ulceration. Often, CNN-associated melanomas appear to have evolved in nonepidermal locations.9 Therefore, early detection of melanoma in association with a giant CNN may be difficult and not recognized until a dermal nodule or metastatic disease appears. The prognosis for patients who develop melanoma in association with giant CNNs is usually grave.9 Unlike melanoma in general, there is no ethnic predilection for melanoma developing in giant CNN. There have been multiple cases of lethal melanoma developing in association with small, medium, large, and giant CNNs in black children.
CNNs are characterized by the presence of nevomelanocytes in the epidermis as well-ordered thèques and/or nevomelanocytes in the dermis, which are present as sheets, nests, cords, and/or single cells. Although histopathologic features are cited as being useful in distinguishing nevi as congenital or acquired, there are no known features with absolute specificity and sensitivity. The histopathologic features of very large CNNs may be divided into nevomelanocytic, neuroid, epithelioid cell and/or spindle cell, blue, and mixed types.
In the nevomelanocytic type of large CNN, the histopathologic appearance may be identical to typical acquired nevi, with nevomelanocytes in the epidermis as well-defined thèques and/or nevomelanocytes in the papillary dermis as sheets, cords, or nests. CNNs are more likely than acquired nevi to have nevomelanocytes in the lower two-thirds of the reticular dermis and to be associated with appendageal and neurovascular structures (Fig. 122-5). There may be preferential involvement of one or more appendageal structures in CNN, such as eccrine ducts, and these structures may be abundant and malformed. Veins may be preferentially involved, with an “inflammatory” appearance showing nevomelanocytes within and around blood vessel walls. Subendothelial protrusion by nevomelanocytes in lymphatic vessels may be prominent. Arrector pili may be malformed, large, and infiltrated by nevomelanocytes. Hair follicles are often quite large and frequently associated with abundant melanin in the hair bulb. Nevomelanocytes in the deep reticular dermis may be distributed as single cells or as a single-file array insinuating among collagen bundles, sheets of cells, or combinations of patterns.
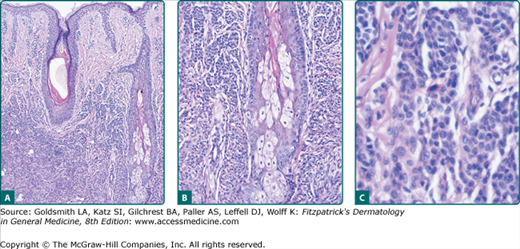
Figure 122-5
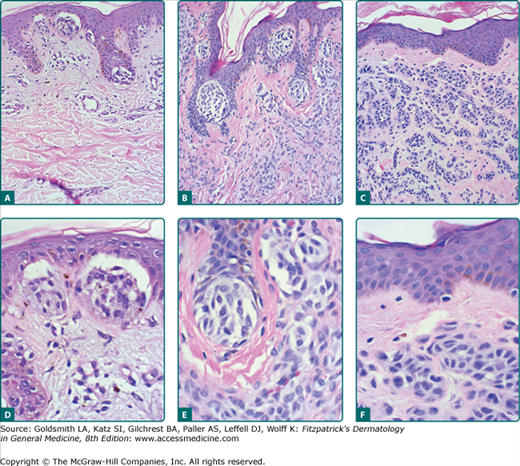
Histopathologic features of congenital nevomelanocytic nevus. Nevomelanocytes in the low-magnification image (A) reveal dense accumulation in the lower two-thirds of the dermis; at medium magnification (B), these cells encroach on dermal adnexal structures (follicular, sebaceous, and eccrine); and higher magnification (C) reveals dense collections of small nevomelanocytic cells.
In the neuroid type of giant CNN, melanocytic elements take on the appearance of Wagner–Meissner corpuscles (lames foliacée), a palisaded arrangement of cells around a cellular mass of homogeneous material (Verocay body), and sheathing of nerves by neuroid tissue (neuroid tubes). The neuroid type of nevus may be responsible for lobulation and redundancy of tissue (pachydermatous or cerebriform appearance) due mainly to the production of connective tissue elements such as reticulin, collagen, and sometimes mucinous stroma. The neuroid type of giant CNN may take on the appearance of a pigmented neurofibroma and may be associated with congenital anomalies of bone (club foot, spina bifida, and atrophy) as well as with a “neurosarcomatous” morphologic variant of melanoma.
In the spindle cell and/or epithelioid cell type of giant CNN, the dermis may be infiltrated in whole or in part by nests or sheets of epithelioid cells and/or spindle cells. Unlike the usual variety of acquired epithelioid cell and/or spindle cell nevus, the epithelioid and spindle cell elements in CNN may involve deep reticular dermis, often intermixing with neuroid elements and ordinary nevomelanocytes. The epithelioid cell and/or spindle cell elements in giant CNN may have atypical cellular and architectural features, making differentiation from melanoma extremely difficult. In some cases, large epithelioid cells may comprise superficial papillary zones of the nevus, and smaller nevomelanocytes may appear in the same lesion in deeper zones of the reticular dermis, with a grenz zone separating the two elements.
In the blue (dermal melanocytic) type of giant CNN, the appearance may be that of a giant blue nevus, or the lesion may have elements of blue nevus (either common or cellular type) with heavily pigmented spindle-shaped melanocytic cells alone or intermixed with nevomelanocytes in the reticular dermis or deeper tissues. There may be some biologic overlap with nevus of Ito/Ota and Mongolian spot.
Unique to the very large CNN is the occasional presence of nevomelanocytes within the substance of muscle, bone, placenta, umbilical cord, cranium, and dura mater. Very extensive CNN may be intermixed with elements of vascular malformation, hemangiomas, increased numbers of mast cells, cartilage, calcification, and even bone. There may be sparse mononuclear cell infiltrates associated with some giant CNNs. In addition to melanoma, associated tumors developing in CNN include schwannoma, neuroid tumor, lipoma, rhabdomyosarcoma, neurofibroma, sebaceous nevus, hemangioma, lymphangioma, and mastocytoma. A possible explanation for these mixed neoplasms containing melanocytic, neuronal, and other elements is that the CNN precursor cell, at least in some cases, is a pluripotent stem cell that has the capacity to give rise to multiple cell types.
In giant CNNs, nevomelanocytes have been found in regional lymph nodes without further evidence of progressive metastatic disease. Whether this deposition occurred during the migration of the cells through the dermis or was secondary to migration from the skin after the neoplasm developed is unknown. The presence of nevomelanocytes in the nodal tissue from these lesions does not imply malignancy (see Section “Nodal Nevi”).
CNNs may also give rise to proliferative nodules that may be difficult to differentiate from melanoma.10
For patients with concern of possible leptomeningeal involvement, magnetic resonance imaging should be considered. For patients with concern of melanoma metastatic, positron emission tomography scan may be considered.
|
The relationship between melanoma and large CNNs is well documented. The risk of melanoma development appears to be proportional to the size of the congenital nevus. The cumulative 5-year risk has been calculated to be 2.3%11 and 5.7%12 in patients with congenital nevi that involve over 5% of the body surface. The lifetime risk of melanoma for patients with very large CNNs has been estimated to be at least 6.3%, based on a questionnaire follow-up study of 151 persons with CNNs examined between 1915 and 1973 in Denmark.13 Melanoma may develop in large CNNs at any time, but the diagnosis of melanoma was established in the first 3–5 years of life in approximately one-half of published cases in which patients ultimately developed melanoma in association with giant CNN.
A causal association between small CNNs and melanoma is more difficult to establish than for large CNNs. When histopathologic features were studied, 6% to 8% of primary melanomas were found to be in contiguity with nevi that had microscopic features characteristic of CNN.14,15 These findings support the concept of melanoma risk even with small congenital nevi, given the expected chance association based on body surface area considerations.16
For cranial, midline, or CNN with multiple satellite lesions, there is a risk of leptomeningeal involvement. Symptomatic leptomeningeal melanocytosis carries a poor prognosis, even in the absence of malignant degeneration.
CNNs have a dynamic evolution during body growth. CNNs at birth usually distort the skin surface at least slightly when assessed by oblique lighting and may become more elevated over time. Surface pigmentation also may change. Lightly pigmented CNNs may become more darkly pigmented, and darkly pigmented CNNs eventually may become less pigmented. CNNs may also develop a halo of depigmentation, potentially heralding spontaneous regression. Loss of pigmentation has been associated with regression of underlying nevomelanocytes and the replacement with sclerosis in some cases. Relatively hairless CNNs at birth may develop long, dark, coarse hair, or may maintain a relatively normal hair density. With few exceptions, CNNs generally expand in direct proportion to growth of a given anatomic zone, although disproportionately rapid area expansion of some congenital nevi may occur during early infancy.6 Lesions in fully grown individuals should remain stable.
The treatment of CNNs, large and small, depends on the perceived risk of melanoma plus cosmetic and functional considerations.
Melanoma may arise in very large CNNs even in the first several years of life. Therefore, excision of very large CNNs should be considered as early as possible, but it is probably prudent to wait until after the first 6 months of life to reduce surgical and anesthetic risks. Management of patients with very large CNNs must be individualized. Extensive involvement of the body surface, with little or no normal skin available for graft sites, may necessitate abandoning efforts at prophylactic excision and accepting lifelong surveillance to detect the earliest signs of malignant change. It may be impossible to remove every nevomelanocyte in very large CNNs, particularly when there is involvement of vital structures or deep anatomic zones. The treatment goal is to remove as much of the nevus as possible while preserving function and improving cosmetic appearance. Other indications for surgical excision of very large CNNs include chronic pruritus, ulceration, and infection. Unlike surgical excision, dermabrasion and other modes of destructive therapy do not address the malignant potential of CNN; nevomelanocytes may still be left behind in the dermis, and the cosmetic results associated with destructive therapy are unpredictable. Melanoma has been reported after dermabrasion of large CNNs.17 “Pseudomelanoma” has been described after laser treatment of a giant CNN.18
The approach to small CNNs needs to be considered carefully. It appears that the risk of melanoma development depends on CNN size, and therefore, smaller lesions appear to have less risk. However, due to the smaller size, complete excision of small congenital nevi may be relatively straightforward, resulting in excellent cosmetic results. Although atypical-appearing CNNs should be considered for immediate excision, careful surveillance without excision may be an option for clinically benign lesions depending on gross appearance, size, location, cosmetic and functional deficits (or improvement) resulting from excision, and general health issues. Given the risk of general anesthesia, for lesions perceived to be at low risk during the first decade of life it is appropriate to consider waiting until the child is old enough to tolerate local anesthesia. All CNNs should be documented at birth, preferably in the form of high-quality photographs that can be used to aid follow-up by parents and physicians. Follow-up is complicated by the natural evolutionary changes that take place in a nevus during body growth (i.e., surface, size, color, and hair), and periodic updates of photographs may be warranted. Suspicious changes in color, surface, or size require urgent evaluation.
There is no known preventive approach to avoid the development of congenital nevi. UV radiation (UVR)-induced mutations clearly play no role in the initial development of the lesions already present at birth. There are no current data on parental exposure that may give rise to congenital nevus formation in their offspring. The role of subsequent UVR exposure for secondary development of melanoma is unclear. However, it seems prudent to protect lesions from unnecessary UVR exposure.
Nevus Spilus
|
Nevus spilus occurs in less than 0.2% of newborns, 1% to 2% of white school children, and 2% of white adults.19 There are no prevalence data in darkly pigmented persons. There does not appear to be a gender predilection.19 When viewed as a risk marker for melanoma, nevus spilus shows a trend for being more common in melanoma cases than in controls. Kopf et al19 detected nevus spilus in 5 (4.8%) of 105 white adults with melanoma compared with 14 (2.3%) of 601 dermatology outpatients.
Nevus spilus may be postulated to develop along similar pathways as a congenital nevus, but instead of the underlying mutation creating a massive accumulation of nevomelanocytic cells in the dermis, the genetic defect creates a field of cells that are susceptible to a secondary event leading to the development of focal individual melanocytic neoplasms within the localized melanocytic hyperplasia. The specific mutations, chromosomal aberrations (mosaicism), and/or dysregulated pathways involved in this process are unknown.
Rarely, nevus spilus may be present at birth. More commonly, the lesion becomes evident during infancy or early childhood. More darkly pigmented, flat, or raised elements are usually present when the lesion is first recognized, but new pigmented elements may appear over time.
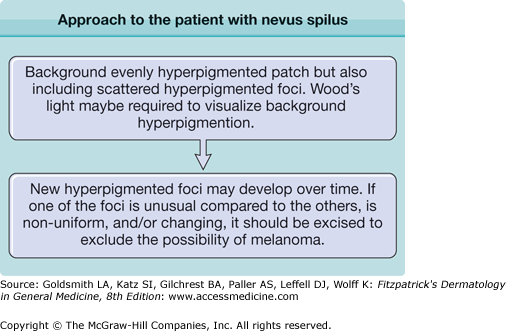
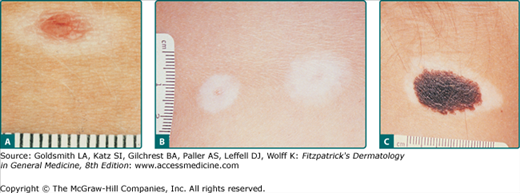
Clinically, the lesion presents as a circumscribed macule/patch of tan pigmentation with features consistent with lentigo or café-au-lait macule including scattered, more darkly pigmented nevomelanocytic (or more hyperplastic) macular and/or papular elements. The tan macular background pigmentation ranges from less than 1 cm to greater than 10 cm in diameter (Fig. 122-7). Although nevus spilus may occur anywhere, lesions have been noted primarily on the torso and extremities. A divided nevus spilus of the eyelid has been reported.20 Lesions may be localized or have a segmental distribution. Giant varieties of nevus spilus may occur.
Nevus spilus has been associated with other anomalies of vascular, central nervous system, or connective tissue origin. Multiple granular tumors and nevus flammeus have been associated with a giant nevus spilus. Large varieties of nevus spilus may be associated with muscle atrophy, other neurological disorders, and phacomatosis pigmentokeratotica.21
The tan background pigmentation usually consists of increased numbers of melanocytes in a lentiginous epidermal pattern. Routine and electron microscopic studies of nevus spilus may demonstrate melanin macroglobules in some cases. The flat, dark elements of nevus spilus may demonstrate foci of increased melanocytic hyperplasia or melanocytic dysplasia (architectural disorder and variable cellular atypia), whereas the raised elements usually contain collections of nevomelanocytes in the epidermis and/or dermis. The neoplastic elements of nevus spilus may also consist of epithelioid and/or spindle cell nevi, nevi with dysplastic features, or blue nevi.
There are no identified molecular tests of value, at least currently.
There are insufficient data to be certain of the natural history of nevus spilus or to comment specifically about its malignant potential; however, caution must be exercised in affected individuals. More than 20 cases of melanoma have been reported to develop in contiguity with nevus spilus, with some cases ending in metastatic disease and death.
In general, nevus spilus is a benign lesion that may develop more spotted elements over time. Once developed, nevus spilus lesions are presumed to persist throughout life, although it is possible that some elements within the nevus spilus or even the entire nevus spilus itself could regress with time. There are no long-term follow-up studies of nevus spilus.
No standard guidelines exist for the management of patients with nevus spilus. Clinical appearance (typical or atypical), history of stability or instability of pigmented elements, congenital or noncongenital onset, perceived risk of developing melanoma, and cosmetic concerns are considerations when determining whether to excise or recommend periodic clinical evaluation for life. Atypical-appearing new and/or unstable elements in nevus spilus should be evaluated by histopathologic examination to exclude melanoma. A nevus spilus with dysplastic or congenital features may have a greater risk of developing melanoma.22,23
Common Acquired Nevomelanocytic Nevus
|
Common (also called typical) acquired nevomelanocytic nevi develop after birth, slowly enlarge symmetrically, stabilize, and after a period of time may regress. The majority of common acquired nevi appear to develop during the second and third decades of life, although some lesions may appear in the first 3–6 months of life.
A number of studies have quantified the number of typical acquired nevi in different age groups. In 432 European whites between the ages of 4 days and 96 years, nevi that were 3 mm in diameter or larger were detected in females and males, respectively, at median numbers of 0 and 2 in the first decade, 10 and 16 in the second decade, 16 and 24 in the third decade, 10 and 19 in the fourth decade, 12 and 15 in the fifth decade, 4 and 12 in the sixth decade, and 2.0 and 3.5 in the seventh through the ninth decades.24 In a series of Australian whites, the average number of nevi per person peaked at 43 for males and 27 for females during the second and third decades, respectively, and decreased to very few in the sixth and seventh decades.25 A similar age-related prevalence rate for nevi has been documented in other countries. A difference in frequency distribution of nevi according to gender is not clear, although most series show a close to equal prevalence in males and females.
The prevalence of acquired nevi varies according to ethnicity. In African blacks, the overall prevalence of nevi (regardless of size) tends to be higher in those with lighter skin versus those with darker skin.26 When prepubertal whites were examined for nevi, a significant association for excess nevi was documented for pale skin, blue or green eyes, blond or light-brown hair, and a tendency to sunburn, but not a tendency to freckle.27 Other studies show variable relationships to these same parameters.
Environmental exposure to UVR appears to be a critical permissive or inciting factor for the development of nevomelanocytic nevi. In an Australian study, nevus density has been shown to increase with increasing sun intensity in the northern parts of the continent.28 Furthermore, the use of UVR-blocking sunscreens has been shown to decrease the number of new nevi in children.29
Genetic factors appear to play a role in the development of nevomelanocytic nevi. The size, frequency, and distribution patterns of acquired nevi tend to aggregate in families. This observation is well documented for atypical nevi in the setting of familial cutaneous melanoma (see Chapter 123) and CNN.
Acquired nevi may be attacked by the patient’s immune system, resulting in the development of a halo nevus (see Section “History”). Of 8,298 whites aged 12–16 years, there were 51 males (1.2%) and 22 females (0.5%) who had one or more halo nevi, for an overall prevalence rate of 0.9%.30 In one large series of halo nevus,31 individuals ranged in age from 3 to 42 years (mean age, 15 years), with the vast majority of cases occurring before age 20 years. The halo phenomenon has been associated with prominent numbers of common nevi, prominent numbers of atypical nevi, and CNN both small and large.
There has been an ongoing debate as to the origin of nevomelanocytic nevi. Although many theories have been proposed, and some supporting data are available, the critical events leading to nevus development remain a mystery. Although data suggest that nevomelanocytic nevi are clonal,32–34 the B-RAF35 mutation that is present in the majority of common acquired (including atypical) nevi appears to be polyclonal36 suggesting that B-RAF mutation is either a secondary event or that nevomelanocytic nevi can incorporate multiple precursors. Melanocytic “precursor” cells have been shown to be present in adult human skin37 and melanocytic stem cells have been identified in the mice.39 It is assumed that these melanocytic stem cells accumulate mutations, and when needed to replenish melanocytes, they produce nevomelanocytes instead. Presumably, these nevomelanocytes then attempt to migrate along normal developmental pathways from the dermis into the epidermis. Dependent on the underlying mutation, some cells might also migrate aberrantly. The defect, timing, and local tissue influences could allow for the creation of a variety of different melanocytic neoplasias.38
The clinical phenomenon of eruptive nevi also further confounds the location of the cell of origin issue. In these patients, there is the abrupt appearance of numerous similar appearing nevi often in the setting of immunosuppression or chemotherapy. Although it is generally assumed that these lesions all directly originate in the skin, drawing a comparison with epidermotropic metastatic melanoma leaves open the possibility of a circulating nevomelanocytic precursor.
Acquired nevi primarily develop during childhood and early adulthood. Although nevi can clearly persist in a stable state for decades, many eventually regress. In a study following patients with dysplastic/atypical nevi, the highest rate of nevus development and nevus regression was present in patients younger than 30 years of age.40 In later adult years, nevus counts are significantly less than those in young adulthood, and the rate of new or growing nevi declines, while melanoma incidence increases. Thus, a growing lesion in an older adult has a greater risk of being melanoma although new or changing moles still significantly outnumber melanomas.40
Spontaneous and concurrent development of scattered nevomelanocytic nevi often similar in appearance has been termed eruptive nevi. Often, these patients have experienced a blistering skin disease, immunosuppression, cytokine administration, or chemotherapy. Eruptive nevi may have a pattern consistent with common acquired nevi, but Spitz nevi and blue nevi have also been described.
Patients may also present with the history of spontaneous development of a zone of depigmentation around a preexisting nevus. This phenomenon has been called a halo nevus. The halo phenomenon may be associated with acquired and CNN as well as with melanoma and nonmelanocytic tumors. This phenomenon often, but not always, indicates the onset of involution and subsequent regression of the melanocytic nevus. Other names for this phenomenon include leukoderma acquisitum centrifugum, Sutton’s nevus, leukopigmentary nevus, perinevoid vitiligo, and perinevoid leukoderma. Time for full evolution to depigmentation of the halo is not known, but patients report the phenomenon occurring over days to weeks, with little change occurring in the halo thereafter. The central nevus may persist or eventually disappear. Disappearance may take months to years. Areas of depigmentation that remain after the central nevus disappears may remain stationary for many years or repigment after months to years. There are lesions in which the nevus does not involute, and repigmentation of the depigmented halo may occur. Halo may be associated with depigmentation at other sites, and the depigmentation may resolve when the CNN is excised (see Fig. 122-10).
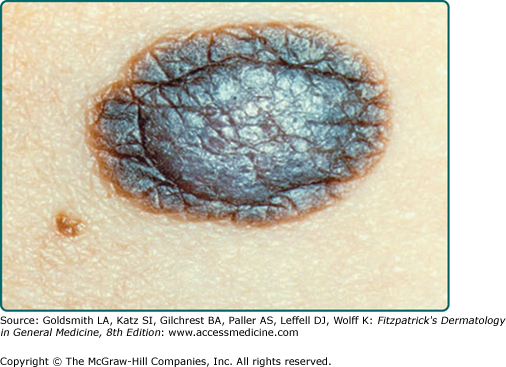
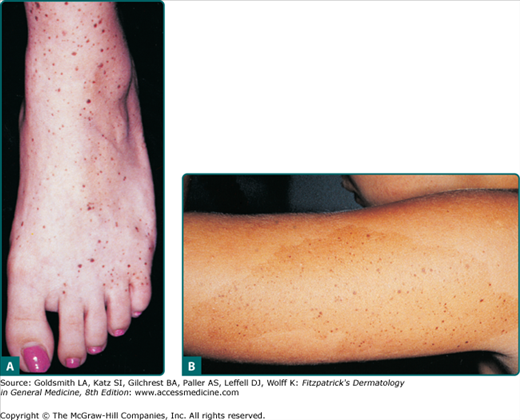
Common acquired nevi vary considerably in their gross appearance. In general, appearance to the naked eye is orderly: the lesions have a homogeneous surface and coloration pattern, round or oval shape, regular outlines, and relatively sharp borders (Fig. 122-9). Common acquired nevi may be papillomatous, dome-shaped, pedunculated, or flat-topped and are usually skin-colored, pink, or brown. More elevated acquired nevi tend to be more lightly pigmented, and flatter acquired nevi tend to be more darkly pigmented. More elevated and less pigmented lesions tend to have a prominent intradermal nevus component, whereas flatter and darker lesions have a more prominent junctional melanocytic or nevomelanocytic component and a less prominent dermal component. Very dark brown and black are unusual colors for common acquired nevi in lightly pigmented people. In contrast, dark pigmentation is usual for common acquired nevi in people who have darkly pigmented skin. Blue, gray, red, and white areas in a nevus are not typical features and ought to be viewed with suspicion. The surfaces of nevi may reveal hair that is less than, equal to, or greater than that of surrounding skin. Hair in nevi may be coarser, longer, and darker than that in surrounding skin (often these lesions reveal congenital features). Lesions on palms and soles are usually hairless. Size, shape, skin markings, and hair quality of nevi in darkly pigmented persons are similar to those in whites.
Figure 122-9
Typical acquired nevomelanocytic nevi. A. Junctional nevus. A uniformly dark brown macule, round in shape, with smooth, regular border. B. Two compound nevi. A uniformly pigmented papule and domed nodule. The upper lesion is flatter and tan with slight central elevation. The lower lesion is older and is uniformly elevated due to an increased intradermal component.
Figure 122-10
Halo nevomelanocytic nevus. A. Acquired halo nevus on the chest of a 16-year-old white boy whose maternal grandmother had melanoma. B. Acquired halo nevi on the back of a 6-year-old white boy who has dysplastic nevi in the hairy scalp and a history of melanoma in his maternal grandmother. Scale in millimeters. C. Halo congenital nevomelanocytic nevus on the neck of a 4-year-old female of a black African-American mother and white father. This child also had scattered areas of vitiligo-like depigmentation in other sites. A 3-year follow-up after excision of the nevus revealed almost total repigmentation of the widely scattered previously depigmented areas.
Dermoscopy reveals a number of diagnostic patterns in common acquired nevi. In general, these lesions reveal a reticular or globular pattern. On the palms or soles, a parallel-furrow, lattice, or fibrillar pattern may be present. Nonuniform patterns and parallel-ridge patterns are worrisome for melanoma.
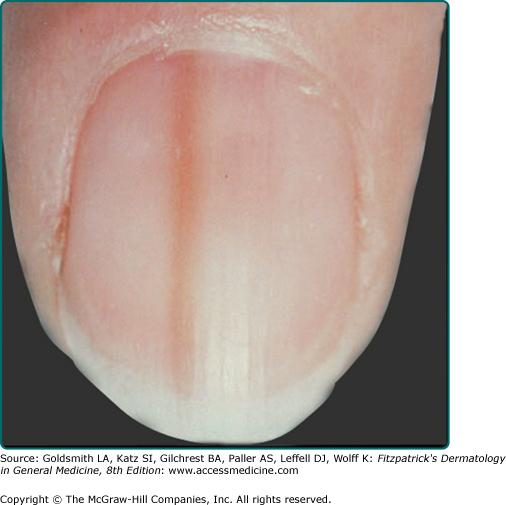
Pigmented nevi of the nail apparatus may be a dark or light brown, extending from the nail matrix to the distal edge of the nail plate (see eFig. 122-9.1); extension of the pigmentation onto the skin of the nail fold or beyond the distal nail groove should be considered suspicious for melanoma. Nevi on palms and soles, even compound nevi, may not distort the skin surface, perhaps because of a thickened stratum corneum in these sites.
The typical halo nevus has a pink or brown central nevomelanocytic nevus surrounded by a symmetric round or oval halo of depigmented skin. The central nevus may be small in typical acquired nevi, or large in acquired atypical or congenital nevi (Fig. 122-10). The halo of depigmentation is variable in size, usually a radial zone 0.5–5.0 cm around the central lesion. White hairs may be seen in hair-bearing depigmented areas. Alopecia areata surrounding a halo nevus has been reported. The number of halo nevi per person may be one or many, multiple lesions occurring in 25% to 50% of patients. For patients who have multiple lesions, only a few nevi are usually affected, but as many as 90 halo nevi have been reported in a single person. Any nevus in any anatomic site may be involved, but the posterior torso is involved most commonly. Lesions are usually asymptomatic. UVR may cause redness and even blistering in the perinevic halo. An atypical gross appearance of the central nevus or an asymmetric halo of depigmentation around a suspicious nevus, particularly in adults, should raise suspicion for melanocytic dysplasia or melanoma. The depigmented halo associated with melanoma tends to be irregular and surrounds the tumor asymmetrically, and the central lesion usually has an atypical appearance. Unfortunately, this appearance is not always the case and a high index of suspicion for melanoma must be maintained in patients who have prominent numbers of atypical nevi (with or without melanoma) and also have halo nevi.
A second process that may provide some insight into factors involved in melanocytic interactions with the immune system is a process that results in the development of an eczematous dermatitis presenting as a red halo around nevi (halo dermatitis, Meyerson’s nevus). Unlike halo nevi, regression of the nevus does not usually occur, and the eczematous changes clear over the course of several months.
For patients with multiple nevi, there is an increased risk for cutaneous melanoma (see Chapters 123 and 124). This risk is further increased in the setting of atypical nevi and/or a personal or family history of melanoma. For patients with eruptive nevi, there may be findings suggestive of a blistering disease or immunosuppression. For patients with halo nevi, the most common associated condition is vitiligo, occurring in 18% to 26% of patients. Halo nevus may be associated with poliosis, Vogt–Koyanagi–Harada syndrome, pernicious anemia, prominent numbers of nevi, atypical nevi, and a personal or family history of melanoma (including ocular melanoma). Relatively large and numerous nevi are common in Turner syndrome and Noonan syndrome.
Nevomelanocytes in the epidermis have a nuclear size similar to or larger than nuclei of epidermal melanocytes. Epidermal nevomelanocytes are arranged in nests surrounded by a smooth perimeter of epidermis, and the epidermis is separated from nevomelanocytes by a retraction artifact (Fig. 122-11). Nevomelanocytes have abundant pale-staining eosinophilic cytoplasm and may have pseudopodic or dendritic extensions that are more evident when enzymatic or immunohistochemical techniques are used for visualization. Nuclei of nevomelanocytes are pale-staining, characterized as vacuolated or reticulated; a nucleolus is usually visible.41 Epidermal melanocytes between junctional thèques of nevomelanocytes in typical nevomelanocytic nevi are disposed as single typical cells, with overall numbers equal to or slightly greater than that in adjacent sun-exposed skin (see Fig. 122-11).42 The overlying epidermis is often normal in appearance but may be thickened in a lentiginous pattern (elongated and club-shaped rete ridges), with an appearance similar to seborrheic keratosis complete with horn cysts or epidermal verrucous hyperplasia similar to epidermal nevus.
Figure 122-11
Histopathologic features of acquired nevi. Junctional nevus (A) and higher magnification (D). Compound nevus (B) and higher magnification (E). Intradermal nevus (C) and higher magnification (F). Well-formed nests of nevomelanocytes are present in the junctional and compound nevi. Sheets and cords of nevocytes are present in the dermis of the compound and intradermal nevi. A grenz zone free of nevomelanocytes is present just below the epidermis in the intradermal nevus (C and F).
The dermal component of nevi has an orderly progression from top to bottom, with larger epithelioid cells above (epidermis and superficial papillary dermis) blending into a pattern of smaller cells in the deeper dermis.
Nevomelanocytes in the epidermis and upper papillary dermis frequently resemble epithelial cells, with an oval or cuboidal shape, a well-outlined cytoplasm that is homogeneous in character, a nucleus not much larger than nuclei of basal keratinocytes, and a visible nucleolus. In the epidermis and superficial dermis nevomelanocytes frequently contain small amounts of melanin. In the middle or deep dermis they are usually smaller than nevomelanocytes in the superficial dermis or epidermis and frequently resemble lymphoid cells. Nevomelanocytes in the deep dermis may be round or oval and often resemble Schwann cells or fibroblasts when they are singly disposed and may be difficult to differentiate from fibroblasts unless they are disposed in sheets, cords, or patchy aggregates (see Fig. 122-11).43 Usually, dermal nevomelanocytes are interposed among collagen bundles, and there is no distinct rim of collagen or retraction artifact between surrounding collagen and cellular aggregates. Nevomelanocytes in the dermis of typical acquired nevi have a monotonous similarity one to another within the same anatomic level and an overall symmetry of architecture from top to bottom and side to side.
Some acquired lesions are found to have congenital features (reviewed in Section “Congenital Nevomelanocytic Nevi”) suggesting that though these features may be present in congenital nevi, they are not limited to those present at birth. Other acquired nevi may be found to have atypical features (see Chapter 123).
Inflammatory cellular infiltrates in typical, stable acquired nevi are usually scanty41 or absent. Melanin-laden macrophages are usually apparent in the superficial papillary dermis of nevi, their number usually proportional to degree of melanin production.41 Blood vessel proliferation, eosinophilic fibrosis, and lamellar fibroplasia (features frequently seen in atypical melanocytic nevi) are usually not prominent in typical acquired nevi. Langerhans cell density overlying typical acquired nevi and atypical nevi is increased compared with adjacent skin.44
Intradermal nevi that have few or no junctional nests frequently have a grenz zone relatively free of nevomelanocytes just below the epidermis (see Fig. 122-11).
Multinucleated nevomelanocytes occur occasionally and may be interpreted as a sign of benignity. Nevomelanocytes in the deep dermis may be disposed within a collagenous framework that is loose, pale, and wavy in formations called neuroid tubes, similar to a neurofibroma; they may be disposed in concentrically arranged whorls resembling Meissner’s tactile corpuscles; and they may be spindle-shaped and embedded in loosely arranged connective tissue (neural nevi). Both neural nevi and neurofibromas show nonspecific cholinesterase activity. Myelin basic protein is detected in various neural tumors but not in melanocytic or nevomelanocytic tumors, and melanosomes are present in nevomelanocytes but not in neurofibromas or nerve tissue. Cutaneous neural lesions may be distinguished from melanocytic tumors by the presence of myelin basic protein and neurofilaments, and the absence of vimentin.
The balloon cell nevus is composed of peculiar foam cells comprising a portion or all of a given lesion. In addition to clear cells with single basophilic nuclei, multinucleated balloon cells and multinucleated giant cells are seen frequently. Electron microscopic studies suggest that vacuolization of nevomelanocytes in balloon cell nevi is due to enlargement and destruction of melanosomes. There appears to be no distinguishing gross features of balloon cell nevi.
The combined nevus refers to the incontiguity association of different types of melanocytic nevi. Most combined blue nevi are found in association with a benign compound nevus (acquired or congenital).
Recurrent melanocytic nevus (pseudomelanoma) is the name given to recurrent lesions after incomplete removal of a benign nevomelanocytic nevus.45 Recurrent nevi are relatively common after superficial destructive procedures (i.e., shave biopsy or dermabrasion). A markedly atypical clinical and histopathologic appearance may accompany this recurrence, making these lesions worrisome for possible melanoma. Clinically, the recurrent nevus is confined to the scar but may be markedly irregular. Histopathologically, recurrent lesions often demonstrate melanocytic hyperplasia in a lentiginous or junctional pattern (often to a greater extent than the original nevus). Moderate nuclear atypia may be present in 12% of recurrent nevi, mitotic figures in 8%, and pagetoid upward migration in 3%, raising concern about the potential biologic behavior of some recurrent lesions.46 It is important to review the original histopathologic specimen when making re-excision and margin decisions.
No definitive and consistent histopathologic features have been described for eruptive nevi.
For halo nevi, the usual histopathologic findings are a central nevomelanocytic nevus associated with a dermal band-like lymphohistiocytic infiltrate and a depigmented zone totally or almost totally devoid of epidermal melanocytes. Immunohistochemical staining—melanoma antigen recognized by T cells (Mart-1), S100 protein, or other melanocytic markers—may help to identify residual epidermal melanocytes or the nevomelanocytes in the inflammatory infiltrate. Lymphocytes in halo nevi appear to be antigenically stimulated, and 80% are T lymphocytes, whereas B lymphocytes are scarce or absent. The T cells appear to belong to the CD8+ phenotype.47
Other findings have been identified in nevi that may suggest modes of regression or elimination of melanocytic elements. Some of the findings include neuroid, fibrous, mucinous, and fatty degeneration.48 Nevomelanocytes in the stratum corneum49 represent transepidermal elimination. Psammoma bodies and amyloid bodies, present occasionally in nevi, also may be related to degeneration or regression. Fibrosis may occur as an age-related phenomenon, whereas true desmoplasia appears to be a reactive process or functional transformation by the nevomelanocytes. Follicular mucinosis has been reported in nevi in at least two cases. Spicules of bone are observed occasionally in nevi, possibly related to reactive metaplasia, trauma, or infection.
Histopathologic artifacts may occur in nevomelanocytic nevi. Shrinkage clefts may resemble lymphatics or vascular spaces, prominent in the midportion of nevi and particularly in areas with hemorrhage. Separation of sheets of nevomelanocytes into parallel rows may be caused by improper cutting. Local anesthesia injection directly into the nevus also may be associated with artifactual changes.
The state of the nevus at the time of excision may also influence the histopathologic appearance. Nevi acutely sunburned or traumatized may have features worrisome for melanoma, and these changes resolve over a short time period.50 Nevi in a rapid growth phase also may have atypical features such as epithelioid cell appearance and pagetoid upward migration, but these observations require corroboration.
Worrisome features for possible melanoma include pagetoid upward migration of cells in the epidermis, atypical features of epidermal melanocytes [including irregularity of size and shape of cells, condensation of chromatin on nuclear membranes, a heterochromatin (clumping) pattern], failure of the cells to “mature” in the deeper dermis, persistence of pigment production in the deep dermis, lack of symmetry, frequent mitotic figures, focal areas of necrosis, and desmoplasia or fibrosis in the dermis.
Special tests are not generally required in the gross and microscopic assessment of melanocytic or nevomelanocytic nevi. However, for patients who have eruptive nevi, given the association with immunosuppression, immunologic tests may be in order. Immunohistochemical profiles may be of value for difficult to diagnose lesions. Low Ki67 activity and loss of HMB45 in dermal (deeper) cells may help confirm a benign diagnosis. Compared to melanoma, common acquired nevi are genomically stable.51 FISH (fluorescence in situ hybridization) or CGH (comparative genomic hybridization) analysis may ultimately be useful in discriminating benign lesions from lesions with a metastatic tendency.
PIGMENTED LESIONS
|
Multiple studies demonstrate that prominent numbers of nevi indicate an increased melanoma risk. Tucker et al52
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree