Diabetes Mellitus
|
Diabetes mellitus (DM) is a major cause of morbidity and mortality in the United States. More than 24 million Americans have the disease1, and approximately 11% ($92 billion) of all health care expenditures in the United States were directly attributable to the medical care of diabetes in 2002.2 Men and women diagnosed with diabetes at age 40 years are expected to lose 12 and 14 life-years, respectively.3 Major studies have shown that tight glycemic control decreases microvascular disease, i.e., retinopathy, neuropathy, nephropathy, however, coronary vascular disease, the major contributor to morbidity and mortality in patients with diabetes, showed no benefit from intensive glycemic control for patients with a known 10-year duration of diabetes. In one large randomized controlled trial with about a third of patients with known coronary artery disease, intensive glycemic control was, in fact, associated with an increase in mortality. Newly diagnosed type 2 diabetes appears to have long-term benefit from similar degrees of tight control.4 New guidelines for glycemic control (HbA1c <7%) attempt to balance this body of evidence. The data and guidelines have been recently reviewed.5 Tight glycemic control may have a beneficial effect on a subset of skin-related, diabetes-associated disorders, but evidence is generally lacking.
Diabetes is characterized by a state of relative or complete insulin deficiency, leading to gross defects in glucose, fat, and protein metabolism. In type 1 diabetes (formerly insulin-dependent DM), an insufficiency of insulin occurs through a gradual, immune mediated destruction of β islet cells in the pancreas, marked by autoantibodies. In type 2 diabetes (formerly noninsulin-dependent DM), chronic hyperglycemia occurs mainly through end-organ insulin resistance followed by a progressive decrease in pancreatic insulin release associated with aging. A fasting blood glucose level of ≥126 mg/dL or a random value of ≥200 mg/dL on two separate occasions confirms the diagnosis of diabetes. Diabetes may also now be diagnosed with an HbA1c level ≥6.5%. A genetic predisposition and a strong association with obesity exist in type 2 diabetes. In both types of diabetes, abnormalities of insulin and elevated blood glucose levels lead to metabolic, vascular, neuropathic, and immunologic abnormalities. Affected organs include the cardiovascular, renal, and nervous systems, the eyes, and the skin.
Nearly all patients with diabetes have cutaneous findings related to their condition, including those listed in Box 151-1. Some diabetes-associated skin conditions are a direct result of the related metabolic changes such as hyperglycemia and hyperlipidemia. Progressive damage to the vascular, neurologic, or immune systems also contributes significantly to skin manifestations. The mechanisms for other diabetes-associated skin conditions remain unknown.
CUTANEOUS FINDINGS IN DIABETES
RELATED FEATURES IN DIABETES
DIAGNOSIS
MANAGEMENT
|
Hyperglycemia leads to nonenzymatic glycosylation (NEG) of various structural and regulatory proteins, including collagen. Although NEG occurs normally with aging, the process is greatly accelerated in diabetes.6,7 NEG leads to the formation of advanced glycation end products (AGEs) that are responsible for decreases in both acid solubility and enzymatic digestion of cutaneous collagen. Disorders such as diabetic thick skin and limited joint mobility (LJM) are thought to result directly from accumulation of AGEs.8 Studies show that the degree of cutaneous AGEs correlates strongly with retinopathy, nephropathy, and other microvascular complications of diabetes.9
Derangements of immunoregulatory mechanisms also occur in diabetes. Hyperglycemia and ketoacidosis diminish chemotaxis, phagocytosis, and bactericidal ability of white blood cells.10 Historically, infections were a major cause of death in the diabetic patient. This has changed dramatically with improved glucose control and antibiotic use. Despite these improvements, certain infections, such as malignant external otitis, necrotizing soft tissue infections, and the devastating disease of mucormycosis, occur more frequently in patients with diabetes.11
Metabolic abnormalities, including hyperinsulinemia, as is seen in early insulin-resistant type 2 diabetes, can contribute to cutaneous manifestations as well. The action of insulin on the insulin-like growth factor-1 (IGF1) receptor appears to mediate the abnormal epidermal proliferation and resulting phenotype of acanthosis nigricans.12 Dysregulated lipid metabolism occurs with diabetes-associated insulin deficiency. The activity of lipoprotein lipase (LPL) is directly dependent on insulin,13 making insulin central to the processing of triglyceride-rich chylomicrons and very-low-density lipoproteins. In insulin-deficient diabetic patients, defective lipid processing can lead to massive hypertriglyceridemia, manifesting in the skin as eruptive xanthomas. Naturally, disorders of lipid processing also play an integral role in the vasculopathies of diabetes.
Macro- and microangiopathy contribute significantly to the cutaneous complications of diabetes. In patients with diabetes, there is increased “leakiness” or vessel wall permeability, decreased vascular responsiveness to sympathetic innervation, and less ability to respond to thermal and hypoxemic stress.10 In combination with arteriosclerosis of large vessels, these microvascular abnormalities contribute to the formation of diabetic ulcers. In addition, a loss of cutaneous sensory innervation occurs with diabetes, predisposing patients to infection and injury. The loss of neuroinflammatory cell signaling plays a causal role in nonhealing, lower extremity ulcers.14 Patients with diabetes who lack lower extremity vibratory perception have a 15.5-fold increased probability of leg amputation.15
The cutaneous disorders associated with DM are characterized in the following section by disorders with evidence for metabolic, vascular, neurologic, or immunologic pathogenesis induced by glucose and insulin abnormalities and by disorders associated with diabetes, but without a clear pathogenesis (see below).
Cutaneous Disorders of Diabetes Mellitus Associated with Metabolic, Vascular, Neurologic, or Immunologic Abnormalities
Acanthosis nigricans is probably the most readily recognized skin manifestation of diabetes. Acanthosis nigricans is common in the general population, and most cases are linked to obesity and insulin resistance. In some cases, increased androgen production is also identified.16,17 Drug-related and idiopathic acanthosis nigricans or familial acanthosis nigricans have been reported.18 In general, though, acanthosis nigricans should be considered a prognostic indicator for developing type 2 diabetes.19 The prevalence of acanthosis nigricans varies among different ethnic groups. In one study, despite similar obesity rates, the prevalence was lower in whites (0.5%) and Hispanics (5%) than in African-American children (13%).17 This finding suggests a possible genetic predisposition or increased sensitivity of the skin to hyperinsulinemia among certain populations.19 Although historical data have emphasized the relationship between acanthosis nigricans and malignancy, a true association is rare. Only when the onset is particularly rapid, the clinical findings are florid, or in the nonobese or nondiabetic adult with acanthosis nigricans is an evaluation for malignancy beyond routine age appropriate screening warranted. In one author’s experience with seeing more than 12,000 patients with cancer, only two developed acanthosis nigricans.20
Advances are taking place in understanding the pathogenesis of acanthosis nigricans. Insulin clearly plays a central role in the presentation of acanthosis nigricans. In a subset of women with hyperandrogenism and insulin resistance with acanthosis nigricans, loss of function mutations in the insulin receptor or anti-insulin receptor antibodies can be found (type A and type B syndrome).21 It is postulated that excess growth factor stimulation in the skin causes the aberrant proliferation of keratinocytes and fibroblasts that results in the phenotype of acanthosis nigricans.16 In states of insulin resistance and hyperinsulinemia, acanthosis nigricans may result from excess insulin binding to IGF1 receptors on keratinocytes and fibroblasts.12 IGF1 receptors are expressed on basal keratinocytes and are upregulated in proliferative conditions.22 Studies show that high concentrations of insulin stimulate fibroblast proliferation through IGF1 receptors in vitro.23 Other members of the tyrosine kinase receptor family, including the epidermal growth factor receptor and the fibroblast growth factor receptor, have been implicated in acanthosis nigricans. Several genetic syndromes [Crouzon and SADDAN (severe achondroplasia with developmental delay and acanthosis nigricans)] with mutations in fibroblast growth factor receptor 3 result in acanthosis nigricans in the absence of hyperinsulinemia or obesity, implicating this growth factor receptor in the pathogenesis of acanthosis nigricans.22 In several reports of acanthosis nigricans associated with malignancy, evidence suggests that transforming growth factor-β released from the tumor cells may stimulate keratinocyte proliferation via the epidermal growth factor receptors.24,25 Support for the role of different growth factors in the pathogenesis of acanthosis nigricans continues to accrue.
In addition to the direct effects of hyperinsulinemia on keratinocytes, insulin also appears to augment androgen levels in women. High insulin levels stimulate the production of ovarian androgens and ovarian hypertrophy with cystic changes.21 Although associated with elevated androgen levels, the acanthosis nigricans in women with polycystic ovarian syndrome (PCOS) does not respond reliably to antiandrogen therapy, implicating the relative importance of hyperinsulinemia over hyperandrogenism in acanthosis nigricans. Several drugs have also been reported to cause acanthosis nigricans, including systemic glucocorticoids, nicotinic acid, and estrogens such as diethylstilbestrol.16
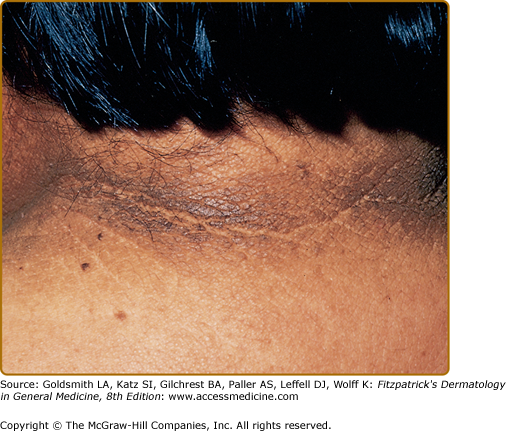
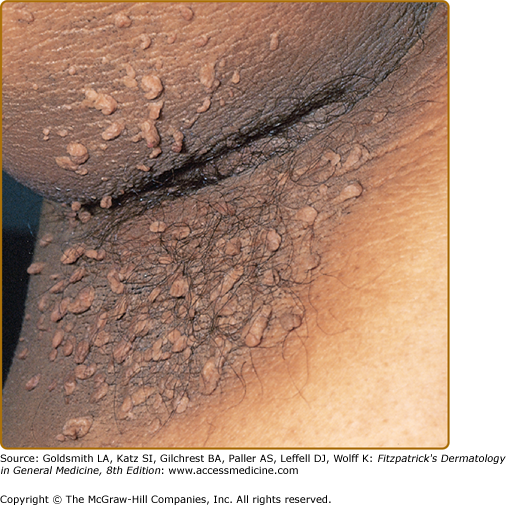
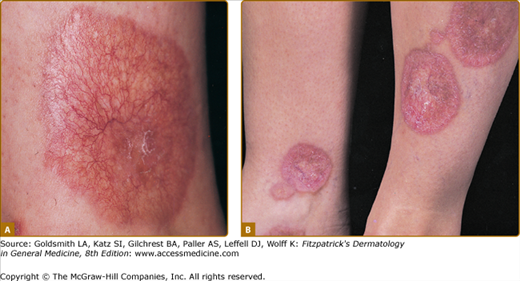
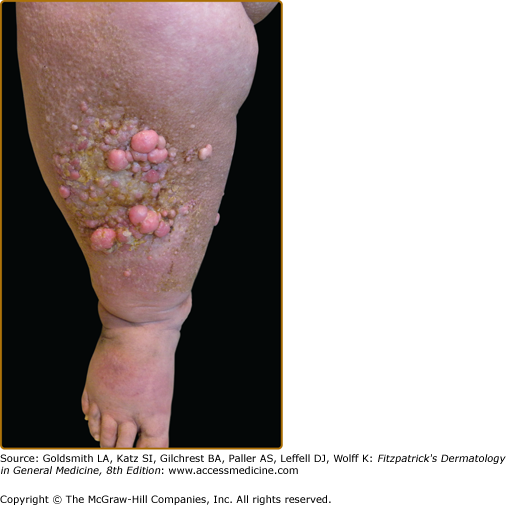
Clinically, acanthosis nigricans presents as brown to gray-black papillomatous cutaneous thickening in the flexural areas, including the posterolateral neck, axillae, groin, and abdominal folds. The distribution is usually symmetric. The affected skin has a dirty, velvety texture. In some cases, oral, esophageal, pharyngeal, laryngeal, conjunctival, and anogenital mucosal surfaces may be involved. In general, however, the back of the neck is the most consistently and severely affected area19 (Fig. 151-1). The development of superimposed acrochordons in involved areas is well described (Fig. 151-2). In particularly florid cases, involvement on the back of the hands over the knuckles and even on the palms can be seen. When the palms are involved, the rugated appearance of the palmar surface has been called tripe palms and is usually associated with acanthosis nigricans seen in the setting of malignancy. In the majority of cases, the most important factor in diagnosing acanthosis nigricans is recognizing the usually associated hyperinsulinemia, which is a known risk factor for type 2 diabetes and the metabolic syndrome.
The histopathology of clinical lesions demonstrates papillomatosis and hyperkeratosis but minimal acanthosis. Hyperpigmentation of the basal layer has been variably demonstrated and the brown color of the lesions is attributed to the hyperkeratosis by most.26,27
Treatment of acanthosis nigricans is generally ineffective. Topical treatment with calcipotriol,28 salicylic acid, urea, systemic, and topical retinoids have all been used with anecdotal success.29 When identifiable, treatment of the underlying cause may be beneficial. Improvement or resolution does occur with weight loss in some obese patients.12,16 Medications that improve insulin sensitivity, such as metformin, have a theoretic benefit. Removal of an offending medication generally results in clearance of the skin.30 In patients with acanthosis nigricans in association with malignancy, there is usually improvement following treatment of the underlying malignancy. The skin finding may present before or after the diagnosis of malignancy is made. When it is associated with malignancy, a tumor of intraabdominal origin, usually gastric, is seen in the majority of cases.31 It has been repeatedly described that patients’ skin improves with chemotherapy and remits with recurrences.
Several specific syndromes are associated with localized thickening of the skin in diabetes. The common underlying pathogenesis involves biochemical alterations in dermal collagen and mucopolysaccharides. The clinical syndromes are a result of increased deposition and improper degradation of these constituents, likely related to the formation of AGEs.
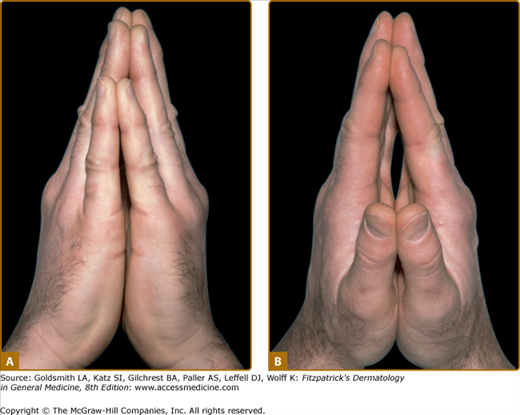
Diabetic LJM, or cheiroarthropathy, presents as tightness and thickening of the skin and periarticular connective tissue of the fingers, resulting in a painless loss of joint mobility. Initial involvement of the distal interphalangeal joints of the fifth digit usually progresses proximally to involve all fingers. Larger joints of the elbow, knee, and foot may be affected. The actual joint space, however, remains uninvolved, so that LJM is not a true arthropathy. This disorder is characterized by the “prayer sign,” which is an inability to approximate the palmar surfaces and interphalangeal joint spaces with the hands pressed together and fingers separated (Fig. 151-3). In addition to joint contractures, the skin may appear thickened, waxy, and smooth with apparent loss of adnexa, resembling skin changes in scleroderma.
Figure 151-3
Limited joint mobility in a 31-year-old male patient with type 1 diabetes mellitus. The patient is unable to approximate the palmar surface of the proximal and distal interphalangeal joints with palms pressed together (known as the “prayer sign”). A. Ulnar view; only fingertips are approximated. B. Radial view; straining to press palms together.
Thirty to fifty percent of adult patients with type 1 diabetes have LJM, and it is common in type 2 diabetes as well. LJM is associated with increased duration of diabetes and poor glucose control.32,33 One longitudinal prospective study showed a 2.5-fold increase in the risk of LJM for every unit increase in the glycosylated hemoglobin.33 In addition, it appears that LJM may be correlated with the presence of microvascular disease.34
Most important, the evidence indicates that intensive insulin therapy is central in prevention and, possibly, treatment of LJM and scleroderma-like syndrome. Long-term tight glycemic control leads to decreased skin AGEs,9 and tight glycemic control is associated with delayed onset and severity of LJM.33 On the basis of improvements in diabetes management, a fourfold reduction in the frequency of LJM over the last 20 years was reported.35 Treatment for LJM is difficult and should focus on tight control of blood sugar as well as physical therapy to preserve active range of motion.
Although the scleroderma-like skin changes can occur independently, they often occur together with LJM in patients with diabetes. The scleroderma-like syndrome is not associated with systemic sclerosis but does correlate with the duration of diabetes, the severity of joint contractures, and retinopathy.36 It seems likely that the historical description of “diabetic hand syndrome” represents a combination of LJM and the scleroderma-like syndrome.
(See Chapter 158)
Recognized in 1970 as a syndrome,37,38 scleredema of diabetes (scleredema diabeticorum) presents with the insidious onset of painless, symmetric induration and thickening of the skin on the upper back and neck. Spreading to the face, shoulders, and anterior torso may occur. The skin retains a nonpitting, woody, peau d’orange quality. Identical changes occur with postinfectious scleredema, usually associated with streptococcal pharyngitis. In scleredema associated with infection, however, the onset is often sudden, and the symptoms usually remit over time. Scleredema diabeticorum affects 2.5%–14% of patients with diabetes.39,40 Scleredema diabeticorum is a disease of long-standing diabetes associated with obesity. Most patients have type 2 diabetes. This disorder has not been reported in children.
The pathogenesis of scleredema diabeticorum is postulated to be unregulated production of extracellular matrix molecules by fibroblasts, leading to thickened collagen bundles and increased deposition of glycosaminoglycans (GAGs, mainly hyaluronic acid). Studies using in vitro fibroblast analysis from involved skin have variably demonstrated increased synthesis of GAGs and type I collagen.41 However, most of these reports involved nondiabetic patients with paraproteinemia and are based on small numbers of cases.
Patients with scleredema diabeticorum may experience decreased sensation to pain and light touch over the affected areas and difficulties with upper extremity and neck range of motion. Extreme cases may result in full loss of range of motion. Unlike in LJM and scleroderma-like syndrome, the presence of scleredema does not correlate with retinopathy, nephropathy, neuropathy, or principal vascular disease.39 However, no large-scale prospective studies have been done. Most patients with scleredema diabeticorum become insulin dependent are difficult to treat, and have multiple complications of diabetes.41 Treatment for scleredema diabeticorum is usually unsuccessful. Case reports describe treatment with radiotherapy, low-dose methotrexate, bath psoralen and ultraviolet A light (PUVA), extracorporeal photopheresis, factor XIII, and prostaglandin E1.38,42,43 Weight reduction and physical therapy to preserve range of motion may be useful.
(See Chapter 135)
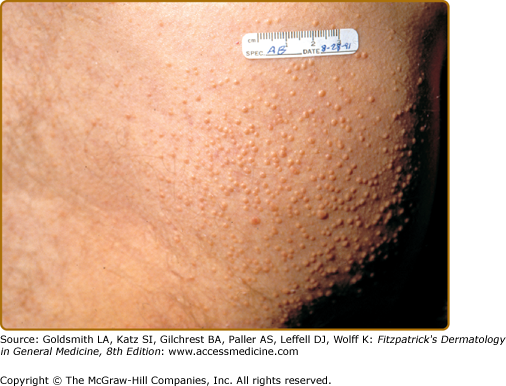
Eruptive xanthomas present clinically as 1- to 4-mm, reddish-yellow papules on the buttocks and extensor surfaces of the extremities (Fig. 151-4). The lesions occur in crops and may coalesce into plaques over time. Although eruptive xanthomas are generally asymptomatic, there is often underlying severe hypertriglyceridemia (>1,000 mg/dL) and potentially undiagnosed diabetes. Histologic and biochemical studies show that lipoproteins (mainly chylomicrons) in the blood permeate cutaneous vessel walls and accumulate in macrophages in the dermis.44 Initially, triglycerides predominate in the skin lesions but, because triglycerides are mobilized more easily than cholesterol, the lesions contain progressively more cholesterol as they resolve.44 Whether this mechanism plays a role in the atherosclerosis of large arteries is unknown.
Insulin is an important regulator of LPL activity. The degree of enzymatic dysfunction and subsequent clearing of serum triglycerides is proportionate to the amount of insulin deficiency and hyperglycemia.13 Clearance of plasma lipoproteins depends on adequate insulin.45 In uncontrolled diabetes, this inability to metabolize and clear triglyceride-rich chylomicrons and very-low-density lipoproteins can lead to plasma triglyceride levels in the thousands. Uncontrolled diabetes is a common cause of massive hypertriglyceridemia.
In addition to eruptive xanthomas, triglyceride levels above 4,000 may cause lipemia retinalis. On funduscopic examination, lipemia retinalis appears as pale pink to white retinal arterioles and venules. The fundus may have a milky hue. Untreated, severe hypertriglyceridemia may also present clinically with abdominal pain, hepatosplenomegaly, pancreatitis, or dyspnea from decreased pulmonary diffusing capacity and abnormal hemoglobin oxygen affinity.46 Treatment of hypertriglyceridemia involves strict dietary fat restrictions and control of the underlying diabetes. LPL activity returns to normal after treatment with long-term insulin or oral glucose-lowering agents.13 The eruptive xanthomas respond rapidly and usually resolve completely in 6–8 weeks.47
In diabetic patients, there is not strong evidence for an increased susceptibility to infections in general, but several skin infections do occur more commonly, with greater severity, or with a greater risk for complications in patients with DM. Joshi et al have reviewed this subject.11
There is extensive research on the pathogenesis of immune dysfunction in diabetes. Although some studies could not detect defects at the cellular level,48 other studies show that leukocyte chemotaxis, adherence, and phagocytosis are impaired in patients with diabetes, especially during hyperglycemia and diabetic acidosis.49 Further studies show that cutaneous T-cell function and response to antigen challenge are also decreased in diabetes.11 Some of the skin infections which occur more commonly or more severely in diabetic patients are shown in Table 151-1.
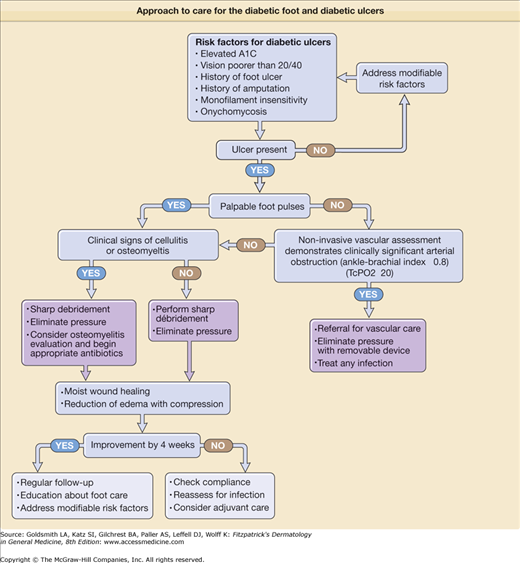
Foot ulcers are a significant problem for patients with diabetes, occurring in 15%–25% of diabetic patients.50 Patients with diabetes have an estimated increased risk of lower extremity amputation that is 10–30 times greater than the general population. Lower extremity ulcers were the proximal cause of amputation in 67 of 80 patients (84%) in a study by Pecoraro et al.51 In diabetic patients with foot ulcers, 14%–24% will eventually undergo amputation.52 The attributable cost of treating a diabetic foot ulcer, excluding the cost of amputation, was estimated to be $28,000 in a 1999 US study.53
Many of the factors previously described in this chapter contribute to the pathogenesis of diabetic ulcers. Peripheral neuropathy, pressure, and trauma are felt to play prominent roles in the development of diabetic ulcers. Neuropathy (associated with uncontrolled hyperglycemia) is one of the major predictors of diabetic ulcers.15 Patients with diabetes also suffer the loss of cutaneous sensory nerves.54 The subsequent diminished neuroinflammatory signaling via neuropeptides to keratinocytes, fibroblasts, endothelial cells, and inflammatory cells may adversely affect wound healing.55 Excessive plantar pressure develops from foot deformities (Charcot arthropathy), as a result of LJM related to NEG, and from callus formation. Ill-fitting shoes and socks were the most common reasons for foot ulcers in a study of 314 diabetic patients with ulcers.56 By wearing running shoes, patients with diabetes had a measurable reduction of calluses in one study.57 Callus formation is a sign of excess friction and often precedes foot ulcers. Once an ulcer develops, peripheral vascular disease and intrinsic wound healing disturbances contribute to adverse outcomes. Known factors associated with foot ulceration in the setting of diabetes include previous foot ulceration, prior lower extremity amputation, long duration of diabetes (>10 years), impaired visual acuity, onychomycosis, and poor glycemic control. Boyko and colleagues have recently published a prediction model for foot ulceration based on the relative contributions of these factors.58
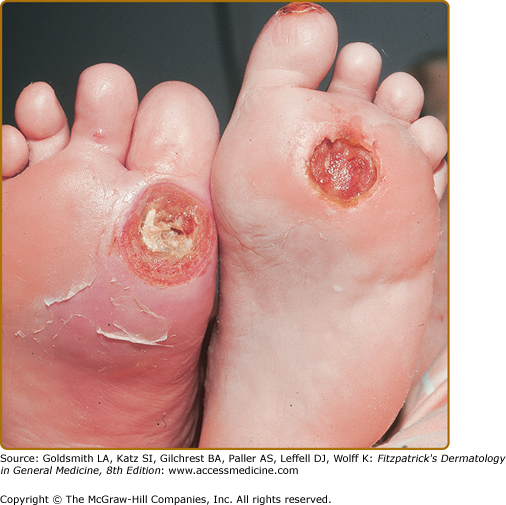
Callus formation precedes necrosis and breakdown of tissue over bony prominences of feet, usually on great toe and sole, over first and/or second metacarpophalangeal joints. Ulcers are surrounded by a ring of callus and may extend to underlying joint and bone (Fig. 151-5). Complications are soft tissue infection and osteomyelitis.
Treatment of diabetic ulcers requires modification of factors that contribute to ulcer formation, for example, stasis dermatitis, leg edema, and skin infection. Standard therapy for neuropathic diabetic ulcers includes debridement, off-loading (often nonweight bearing), moist wound care, and protective dressings (Fig. 151-6).
There has been substantial interest in developing adjunctive therapies for diabetic ulcers, including growth factors and skin-replacement products, but data have not supported their use as a replacement for standard wound care. Recombinant platelet-derived growth factor for the topical treatment of diabetic foot ulcers demonstrates a modest benefit if used with adequate off-loading, debridement, and control of infection.59 A large multicenter, clinical trial of a bilayered living skin equivalent60 showed 56% healing at 12 weeks as compared with 38% healing for standard care. The most favorable published results for a monolayered living skin equivalent and platelet-derived growth factor show roughly comparable improvement in healing to that reported for bilayered living skin equivalents when each is compared with standard care or placebo.60 These technologies await definitive analysis of cost effectiveness compared with standard older approaches. While studies available do not support the routine use of these biologic approaches they may have a role in the treatment of large ulcers (>2 cm) or ulcers poorly responsive to standard therapy. A recent meta-analysis makes the points that typically a higher percentage of ulcers heal during a 12-week study period with biologic products, but analysis of cost effectiveness is made difficult by differences in study designs, short duration of studies, different cost structures, the absence of quality of life measures and pharmaceutical funding of the primary studies and analysis.61
Ulcer prevention is the most important intervention physicians and other health care professionals can provide for diabetic patients. An excellent review of ulcer prevention was published by Singh et al.50 Optimizing glycemic control is well supported to prevent the neuropathy that is so intimately associated with foot ulceration. In a recent study, the risk of a foot ulcer increased in nearly direct proportion to every 1% increase in hemoglobin A1C.58 Foot examination should be part of every patient visit if diabetes is on the problem list. Failure to perceive touch by a Semmes-Weinstein 10-g monofilament means a patient lacks protective sensation in the foot tested. If tinea pedis is present, it should be treated to prevent the associated skin barrier disruption. Smoking cessation should be encouraged. Patients should be counseled about the importance of daily foot care (Box 151-2).
|
Specialized ulcer care teams have published impressive results on the prevention and healing of ulcers.62 This multidisciplinary team approach is becoming more important in the treatment of diabetic ulcers in large population centers. Patients with a history of ulceration are at high risk for reulceration (34% at 1 year, 61% at 3 years, and 70% at 5 years).56 Intensifying education (formal foot care classes) and prevention efforts in this group along with lifelong surveillance is required.
Disorders Associated with Diabetes Mellitus But of Unknown Pathogenesis
Coined by Urbach in 1932 as necrobiosis lipoidica diabeticorum, this disorder was named after characteristic histologic findings and was first described in patients with diabetes. Because not all patients have concurrent diabetes, the shortened term, necrobiosis lipoidica (NL) is preferred. Epidemiologic data show that the mean age of onset is around 30 years, with women representing three times more cases than men.63 The most often quoted statistics concerning the association of NL with diabetes are from a 1966 retrospective study at the Mayo Clinic. Of 171 patients with NL, two-thirds had diabetes at diagnosis, and another 5%–10% had glucose tolerance abnormalities.63 However, in a 1999 study of 65 patients with NL, only 11% had diabetes after 15 years of follow-up.64 Conversely, the prevalence of NL has been found to be only 0.3%–3.0% in patients with diabetes.63 Although lacking full concordance, NL definitely has a strong association with diabetes and remains a valid marker of the disease. The pathogenesis of this skin disease is unclear. Many etiologic mechanisms have been proposed and reviewed.65 Evidence suggests that the degree of hyperglycemia and diabetic control does not correlate with the presence of NL.66
Classically, NL presents with one to several sharply demarcated yellow–brown plaques on the anterior pretibial region (Fig. 151-7). The lesions have a violaceous, irregular border that may be raised and indurated. Initially, NL often presents as red–brown papules and nodules that may mimic sarcoid or granuloma annulare (GA). Over time, the lesions flatten, and a central yellow or orange area becomes atrophic, and commonly telangiectasias are visible, taking on the characteristic “glazed-porcelain” sheen. Aside from the shins, other sites of predilection include ankles, calves, thighs, and feet. Fifteen percent of patients develop lesions on the upper extremities and trunk that tend to be more papulonodular. Although pain and pruritus have been reported, most lesions are asymptomatic. Anesthesia of the plaques does occur.67
Figure 151-7
Necrobiosis lipoidica. A. A single orange plaque with atrophy of the overlying epidermis and arborizing telangiectasias is seen on the lower leg of a juvenile with diabetes mellitus; the crust marks an area of early ulceration. B. Older lesions with striking central atrophy involving both the dermis and epidermis.
The clinical course is often indolent, with spontaneous remission in less than 20% of cases.10 Over time, the plaques tend to stabilize, and formation of new lesions tapers off. However, the possibility of ulceration, a poor spontaneous remission rate, and cosmetic concerns lead patients to seek treatment. Ulceration, the most serious complication, occurs in approximately 13%–35% of cases on the legs.63,65 A few cases of squamous cell carcinoma arising in chronic ulcerative lesions of NL have been reported.68 Multiple reports document the association of NL with GA and sarcoidosis.69
Treatment for NL is disappointing. At this time, only case reports and small, uncontrolled trials provide the basis for treatment decisions. Early application of potent topical glucocorticoids might slow progression.65 Although some authors63 report improvement with intralesional injection of glucocorticoids to the active border, the risk of ulceration with this treatment modality should be considered. A few case reports and one series of six patients70 showed benefit with short-term systemic glucocorticoids. Aspirin and dipyridamole have produced variable results.65 Anecdotal reports exist that support the use of topical retinoids and topical PUVA. Treatment with fumaric acid esters in 18 patients was reported to improve lesions clinically and histologically.71
Given the generally benign nature of the lesions, physicians should consider the adage “do no harm.” Focus should be on prevention of ulcers. When ulceration occurs in patients with NL, the same wound care principles apply as for all diabetic ulcers. Healing of ulcerated lesions with cyclosporine has been demonstrated in several patients.72 Surgical excision down to fascia and split-thickness skin grafting remain as the last resort for very recalcitrant ulcers in NL.73
(See Chapter 44)
The association of GA with diabetes is weaker than that of NL. Although most patients with GA are in good health, without underlying systemic illness, an association with diabetes is supported in the literature. Dabski and Winkelmann74 found diabetes in 10% of 1,353 patients with localized GA and 21% of 100 patients with generalized GA. An additional small retrospective case-control study showed an increased prevalence of diabetes among patients with GA (18%) as compared with the prevalence in age-matched controls (8%).75 Furthermore, there is an impression that diabetes is found more frequently in patients with adult onset GA and in those with generalized or perforating GA, and that these patients tend to experience a more chronic, relapsing course of GA. The pathogenesis is unclear.
Clinical findings and treatment of GA are discussed in Chapter 44.
Atrophic skin lesions of the lower extremity, or shin spots, were first characterized and proposed as a cutaneous marker for diabetes in 1964.76 Shortly after, Binkley coined the term diabetic “dermopathy” to correlate the pathologic changes with those of retinopathy, nephropathy, and neuropathy77 Since that time, controversy has existed about the disorder’s etiology, specificity for diabetes, and association with other microangiopathic complications of diabetes.
The prevalence of shin spots in ambulatory patients with diabetes varies. In a population-based study from Sweden, diabetic dermopathy was found in 33% of patients with type 1 diabetes and in 39% of patients with type 2 diabetes, compared with 2% of controls.78 In other studies, the prevalence rate for individuals without diabetes ranges from 1.5% for healthy medical students to 20% for a group of nondiabetic endocrine patients.79 Diabetic dermopathy occurs more often in patients with an increased duration of diabetes and is more frequent in men.79
It is likely that the lesions are related to antecedent trauma. Lithner80 induced diabetic dermopathy on the legs of diabetic patients with heat and cold injury, whereas nondiabetic control subjects healed without residual change. When questioned, most patients think that the changes are caused by injury, but they are often unable to detail preceding trauma.
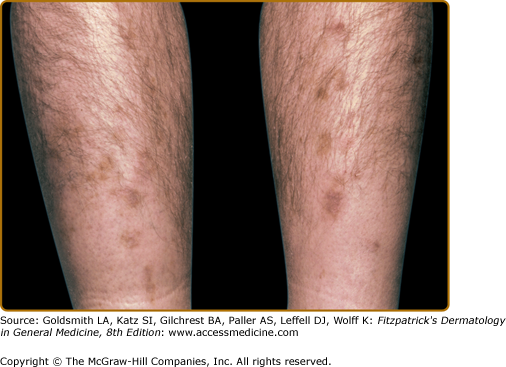
Diabetic dermopathy presents as small (<1 cm), atrophic, pink to brown, scar-like macules on the pretibial areas (Fig. 151-8). The lesions are asymptomatic and clear within 1–2 years with slight residual atrophy or hypopigmentation.77 The appearance of new lesions gives the sense that the pigmentation and atrophy are persistent.
An association seems to exist between diabetic dermopathy and the more serious complications of diabetes. In a study of 173 patients with diabetes, the incidence of shin spots correlated with the duration of diabetes and the presence of retinopathy, nephropathy, and neuropathy.81 Diabetic dermopathy does not, however, correlate with obesity or hypertension in patients with diabetes.79 No treatment is necessary for the individual atrophic tibial lesions. They are asymptomatic and are not directly associated with an increase in morbidity.
(See Chapter 69)
The acquired perforating disorders comprise an overlapping group of disorders characterized by transepidermal elimination or “spitting” of altered dermal constituents. Included in this group are Kyrle disease, reactive perforating collagenosis, perforating folliculitis, and elastosis perforans serpiginosa.82 These disorders and their treatment83 are fully described in Chapter 69.
Clinically, these lesions appear as pruritic, keratotic papules mainly on the extensor surfaces of the extremities. Papules and nodules with a perforating component may also occur on the trunk and face. Many are follicular and contain a prominent central keratotic plug. The papules may be grouped, or coalesce to form verrucous plaques. Treatment for the perforating disorders is usually unsuccessful. Retinoic acid, topical glucocorticoids, and PUVA are partially successful.83
The abrupt, spontaneous development of blisters on the lower extremities without other demonstrable cause is a rare characteristic skin manifestation of diabetes. The pathogenesis of diabetic bullae is unknown. Patients with bullosis diabeticorum (BD) do not have a history of antecedent trauma or infection. One study found a decreased threshold to suction-induced blister formation in patients with diabetes.84 Reduced suction blister time is also observed with increasing age in nondiabetic subjects.85 Although a history of antecedent trauma is not elicited, this finding suggests a role of increased skin fragility in diabetic bullae. Perhaps the formation of AGEs leads to increased fragility.
BD is characterized by the abrupt onset of bullae on the lower extremities, usually the toes, feet, and shins, arising in normal skin. Occasionally, the distal upper extremities are involved. The blisters are usually painless and not pruritic. Healing occurs within 2–5 weeks and rarely leaves scarring. The condition may recur as successive crops of bullae over many years. Studies of affected individuals excluded other blistering skin disorders, and revealed no abnormalities of porphyrin metabolism.84 Histopathologic examination of the bullae shows an inconsistent level of separation varying from intraepidermal to subepidermal.85 No immunopathologic features are consistently observed (Box 151-3).
DIFFERENTIAL DIAGNOSIS
LABORATORY TESTS
|
BD runs a benign course without involvement of large body surface areas. The only serious complication is that of secondary infection, which should be managed with culture and appropriate antibiotics if suspected. Otherwise, therapy is supportive. The real importance of this disorder is that of correct diagnosis because several of the blistering skin diseases have a high rate of morbidity and require potentially toxic systemic treatments. Even a negative workup is important. The patient should be educated, reassured, and good wound care implemented.
Obesity is characterized by excess fat mass and defined according to the body mass index (BMI, kg/m2), a calculation based on weight and height [BMI = body weight (in kg) ÷ square of stature (height in meters)]. BMI is correlated with body fat and the WHO defines overweight as a BMI of >25 and obesity as >30 kg/m2. For Americans the risk of becoming overweight or obese in adulthood is high. As part of the National Health and Nutrition Examination Survey (NHANES) in 2003–2004, 17.1% of children and adolescents were overweight and 32.2% of adults were obese. Approximately 30% of nonhispanic white adults were obese as were 45.0% of nonhispanic black adults and 36.8% of Mexican American. It was also noted that obesity increased in the adult population with age.86
Contributing factors to obesity are nutritional choices, activity and exercise, medications, and rarely one of several endocrine disorders. In the setting of nutrient excess and weight gain, numerous comorbid factors occur that may contribute to further weight gain by increasing energy intake or decreasing energy expenditure. These include, inflammation and insulin resistance, depression and emotional eating, degenerative joint disease, obstructive sleep apnea, gonadal dysfunction, vitamin D deficiency, among others. Familial studies suggest a strong genetic basis for human obesity. The genetics of obesity are complex, though most human obesity is likely polygenic, multiple single genes have been identified as key regulators of body adiposity. Three of these genes [(1) leptin, (2) pro-opiomelanocortin (POMC), and (3) agouti-related protein (AgRP)] with dermatologic relevance are discussed in detail below.
The hormone leptin is secreted by adipose tissue in proportion to total body fat. Leptin regulates energy homeostasis, neuroendocrine function, and metabolism.87 Rare cases in humans have shown that leptin deficiency causes extreme obesity, hyperphagia, diabetes, neuroendocrine abnormalities, and infertility, all of which can be reversed by administration of exogenous leptin. However, most obese humans are leptin resistant, have high circulating levels of leptin and pharmacological leptin administration has not proven to be a successful weight-loss strategy. Leptin resistance appears to be at the hypothalamic leptin receptor or downstream.87 Congenital and acquired forms of lipoatrophy (HIV or HAART associated) are characterized by low leptin levels and metabolic abnormalities including insulin resistance, hyperlipidemia, and fatty liver. Leptin treatment in these patients can improve insulin resistance, lipid abnormalities, and fat distribution.88,89
Leptin stimulates the hypothalamic melanocortin pathway including hypothalamic neurons expressing POMC. Cleavage of POMC results in peptide agonists for all five homologous melanocortin receptors. The melanocortin 1 receptor (MC1R) is expressed on melanocytes and mutations in MC1R are known to cause red hair and fair skin.90 Inactivating mutations in MC4R and to a lesser degree MC3R are associated with obesity. Studies estimate that inactivating mutations in MCR4 account for up to 6% of all severe cases of early onset obesity. Further emphasizing the importance of the POMC pathway is the finding that homozygous loss of function of POMC (complete POMC deficiency) produces obesity, pale skin, and red hair.91
AgRP is an endogenous hypothalamic melanocortin receptor antagonist, which causes obesity when overexpressed. Though studies have found high serum AgRP levels in obese men,92 the role of AgRP in common obesity is unclear. The gene is closely related to agouti, a skin pigmentation gene, which causes yellow coat color and obesity when overexpressed in mice.
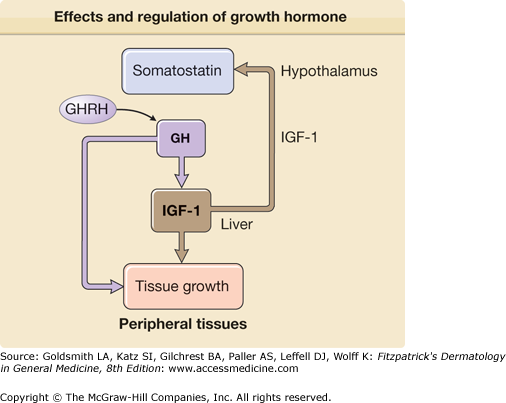
The hormonal regulation of obesity is similarly complex and several gut hormones are likely involved in the regulation of food intake. The gastric derived, appetite stimulating hormone ghrelin impacts obesity and diabetes by modulating body weight, insulin secretion, and gastric motility.93 Serum ghrelin levels rise before meals and are thought to promote food intake. Sharp rises are seen in states of starvation and after weight loss in obesity. This likely contributes to weight regain. Interestingly, postgastric bypass surgery patients have altered ghrelin secretion and this may be one of the reasons for the long-term success of this surgical treatment for obesity.94 Ghrelin receptors are located in the pituitary and hypothalamus and stimulate growth hormone (GH1) release and regulate energy expenditure93 (see Fig. 151-15). Several ghrelin antagonists are in development for the treatment of obesity, metabolic syndrome, and diabetes.
Physiologic changes in the skin related to obesity include alterations in epidermal barrier function,95,96 increased sweating along with larger skin folds, increased skin surface pH in intertriginous areas,97 poor lymphatic drainage (eFig. 151-8.1), impaired wound healing in animal models,98 and impaired responsiveness of the microvasculature.99 Several reviews regarding obesity and dermatology have been published.100,101 The cutaneous disorders seen in obesity are listed in Box 151-4. Detailed discussion of findings and treatments for these disorders are found in the appropriate sections of the text.
METABOLIC
INFECTIOUS
MECHANICAL
MISCELLANEOUS
|
It is now well recognized that the presence of abdominal—central rather than subcutaneous—obesity (more than just increased BMI) is associated with insulin resistance, hyperlipidemia, hypertension, and vascular inflammation (Box 151-5). The coexistence of these disorders increases the risk for diabetes and cardiovascular disease and has been called the metabolic syndrome. It is not clear that the metabolic syndrome confers risk beyond that of the individual components but because the traits co-occur, those with one trait are likely to have others. The most important therapy is weight reduction and exercise along with adequate control of cardiac risk factors.
|
The cornerstones of treatment for overweight and obesity are dietary changes, increased physical activity, and behavioral modification. In high-risk patients with comorbid conditions, pharmacologic therapy (sibutramine or orlistat) can be considered as an additional intervention. In clinically severe obesity, surgical therapies may be appropriate. All successful patients will require long-term nutritional adjustments that reduce caloric intake. The role of liposuction in weight loss has been studied and despite the removal of a large volume of subcutaneous adipose tissue there was no improvement seen in the metabolic risk factors associated with obesity.102,103
|
Worldwide estimates of the prevalence of goiter (enlargement of the thyroid gland) range from 200 to 800 million affected people. The majority of these worldwide cases are secondary to iodine deficiency. However, in industrialized countries where salt is routinely iodized, the main causes of goiter are autoimmune thyroiditis and nodular thyroid disease. The overall incidence of hyperthyroidism in the US population is estimated at approximately 1%, but may be four to five times higher in older women.104 Graves disease accounts for 60%–80% of all cases of hyperthyroidism and was first described by Caleb Parry in 1825 (and later by Robert Graves in 1835) as an association between goiter, palpitations, and exophthalmos.105 Although the incidence of Graves disease is similar between whites and Asians, it appears to be lower in blacks.105 Toxic adenoma and toxic multinodular goiter refer to the hyperplastic growth of thyroid tissue that is functioning independent of TSH regulation, resulting in increased levels of thyroid hormone. Toxic multinodular goiter has been reported to be more common in areas of low iodine intake, particularly in patients older than the age of 50 years.106 Population-based screening with laboratory testing found hypothyroidism in 4.6% of the US population, although >90% of individuals with tests consistent with hypothyroidism were normal clinically.104
The majority of thyroid disease is acquired, but thyroid disease can also be congenital. Congenital hypothyroidism is the most common treatable cause of mental retardation, and occurs in up to 1 in 3,000 neonates worldwide due to either an absent or anatomically defective gland, inborn errors of thyroid metabolism, or iodine deficiency.107 Congenital hyperthyroidism is much less common, and although 0.2% of pregnant women have Graves disease, only about 1% of the resultant neonates will have hyperthyroidism, with most cases resulting from the transfer of maternal thyroid-activating autoantibodies.108
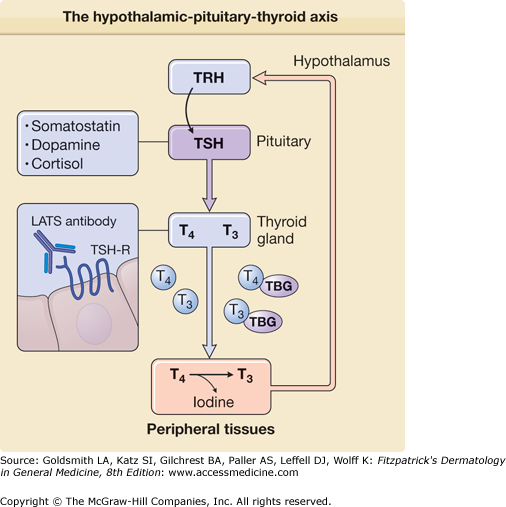
The metabolic regulation of every cell in the body relies on thyroid hormones, which is synthesized primarily in the thyroid gland. Hyperthyroidism (also known as thyrotoxicosis) results from the excess levels of thyroid hormones with resultant hypermetabolism, whereas hypothyroidism (or myxedema) is characterized by hypometabolism secondary to diminished levels of thyroid hormone. Thyroid hormones act on target tissues by activating cytoplasmic receptors that subsequently translocate to the nucleus and activate specific thyroid-responsive genes.109 Thyroid hormone synthesis is heavily dependent on iodine and, consequently, changes in iodine intake can result in both hyperthyroidism and hypothyroidism.
Synthesis of thyroid hormone is regulated by the pituitary via release of TSH in response to hypothalamic release of thyrotropin-releasing hormone (TRH). TSH acts on the thyroid gland by binding a G-protein-coupled receptor (TSHR) to trigger thyroid hormone synthesis and release. Aberrant activation of TSHR is thought to underlie the hyperthyroidism seen in Graves disease, where almost all patients have long-acting thyroid-stimulator autoantibodies that bind and activate TSHR.110 Release of TSH is, in turn, stimulated by TRH, which also acts via a G-protein-coupled receptor. Administration of recombinant TRH followed by measurement of the response in TSH levels provides a useful test for distinguishing whether hypothyroid disease results from dysfunction of the hypothalamic–pituitary axis or the thyroid gland. Levels of TSH and TRH are both tightly controlled by circulating levels of thyroxine (T4) and triiodothyronine (T3), which provide feedback regulation (eFig. 151-8.2). Persistent stimulation of the thyroid leads to the hypertrophy of the gland, known as goiter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree