Dermatitis Herpetiformis: Introduction
|
Epidemiology
Dermatitis herpetiformis (DH) is characterized by an intensely itchy, chronic papulovesicular eruption that usually is distributed symmetrically on extensor surfaces. The disease can be clearly distinguished from other subepidermal blistering eruptions by histologic, immunologic, and gastrointestinal criteria. The prevalence of DH in various Caucasian populations varies between 10/100,000 and 39/100,000 persons.1–3 Some reports suggests a 1.5:1 male to female ratio of DH patients. It may start at any age, including childhood; however, the second, third, and fourth decades are the most common. After presentation, DH persists indefinitely in most patients, although with varying severity. Two long-term studies of immunologically verified patients have suggested that the disease in approximately 10–12% of DH patients eventually remits.4,5
Etiology and Pathogenesis
In 1884, Louis Duhring first described the clinical features and natural history of a polymorphous pruritic disorder that he called dermatitis herpetiformis; however, the critical elements in the pathogenesis of DH remained unknown until the 1960s.6 In 1966, Marks et al first noted a gastrointestinal abnormality in patients with DH.7 Shortly thereafter, it was shown that the lesion was reversible by avoidance of the dietary protein gluten.8,9 Initially, the intestinal abnormality was thought to be present in 60%–75% of DH patients. However, this view has been modified in two ways. First, the diagnostic criteria for DH have been delineated more precisely, and second, it can be shown that certain patients without apparent gastrointestinal pathology can be “induced” to develop gastrointestinal lesions by subjecting them to a large gluten intake; such patients have been said to have latent celiac sprue.10 Thus, most patients with DH have a gastrointestinal abnormality similar (if not identical) to celiac disease, however minimal that may be when the patient is ingesting a normal gluten load. These studies have all confirmed that gluten, a protein found in wheat, barley, and rye, plays a critical role in the pathogenesis of DH. Oats, long thought to contain gluten and play a role in inducing DH lesions, have been shown to be devoid of toxicity in patients with DH.11,12 As in celiac disease, there is an increased density of small bowel intraepithelial T cells with a γ/δ T-cell receptor in the jejunum of patients with DH.13 The finding that T-cell lines from patients with DH produce significantly more interleukin 4 (IL-4) than those from patients with GSE and that gut biopsies from symptomatic patients with isolated GSE showed increased expression of interferon-γ suggests that different cytokine patterns may play a role in the varied clinical manifestations of these two diseases.14,15 Systemic evidence of the gut mucosal immune response has also been found in the serum and the skin of patients with DH. Patients with DH on regular gluten-containing diets have been found to have increased serum IL-2 receptor levels and serum IL-8 levels, increased endothelial cell E-selectin expression in skin, and an increased expression of CD11b on circulating neutrophils.16–18 These systemic manifestations of the gut mucosal immune response may play a role in creating the proinflammatory environment in the skin necessary for the development of skin lesions. The GSE seen in DH patients probably relates to the immunoglobulin (Ig) A deposits that are found in the skin of these patients, although a direct relationship has not been demonstrated. Patients with a clinical picture consistent with DH and partial IgA deficiency have been reported.19
In 1999, Dieterich et al identified antibodies to tissue transglutaminases (Tgases) in the sera from DH patients.20 Distinguishing various types of Tgases enabled Sardy et al in 2002 to demonstrate that epidermal Tgase is the dominant autoantigen in DH.21 This was confirmed by a study of nine DH patients by Donaldson et al demonstrating that the dermal deposits of epidermal Tgase colocalized with cutaneous deposits of IgA, in the papillary tips.22 Because epidermal Tgases were strongly expressed in the upper epidermis, the authors suggested that in regions of trauma they may diffuse through the basement membrane after release from epidermal keratinocytes. Epidermal Tgases were also found in uninvolved skin at least 5 cm away from the lesion suggesting additional factors involved in the production of DH lesions.22
It is also known that patients with both GSE and DH have circulating IgA antibodies directed against Tgases.20,23 There appears to be a predilection for these circulating IgA autoantibodies to bind to epidermal Tgase in DH, whereas the predilection is for autoantibodies to bind tissue Tgase in patients with isolated GSE.21 The precise role of the circulating IgA antiepidermal Tgase in the development of skin lesions in patients with DH is not known. However, children with celiac disease have lower levels of circulating IgA antiepidermal Tgase when compared to adults with celiac disease, whereas levels of circulating IgA antibodies against tissue Tgase are not significantly different between children and adults with celiac disease.24 This observation has led to the hypothesis that epitope spread over time results in the development of IgA antiepidermal Tgases and that this late onset of IgA antiepidermal Tgase antibodies may play a role in the typical development of DH in the second to third decade of life.24
The mechanism whereby the IgA antiepidermal Tgases bind to skin in patients with DH is not fully understood. One long-standing hypothesis has been that IgA-containing circulating immune complexes are responsible for the IgA deposits in DH skin. The recent discovery of IgA antiepidermal Tgase antibodies has led to the suggestion that IgA–epidermal Tgase immune complexes may be depositing in the skin of DH patients. However, only a minority of DH patients have been found to have IgA and epidermal tissue Tgase deposits colocalized in a perivascular pattern.22,25 In addition, perivascular neutrophil deposits that are typically found with the perivascular deposition of immune complexes rarely occur in patients with DH.26 These findings suggest an alternative hypothesis that IgA antiepidermal Tgase may directly bind in the skin to epidermal tissue Tgase. Direct antigen–antibody binding of IgA antiepidermal Tgases to skin also appears unlikely to represent the complete mechanism, since IgA deposits in DH skin cannot be removed using typical techniques for eluting antibody bound directly to antigen. It is possible that the IgA antiepidermal Tgases bind initially via antigen–antibody interactions and that ability of Tgases to cross-link proteins results in the IgA cross-linking to dermal proteins resulting in the stable, long-lasting IgA deposits seen in the skin of patients with DH. This hypothesis awaits confirmation.
Whether the IgA skin deposits play a role in the pathophysiology of blister formation is not known. The finding of IgA and complement in almost all skin sites, not only in lesional skin, makes one postulate that if IgA (either alone or as a part of an immune complex) does play a role, additional factors are still needed to explain the initiation of lesions. Takeuchi et al have demonstrated that minor trauma to skin results in increased expression of IL-8 and E-selectin, both of which may predispose to a neutrophilic inflammatory infiltrate.27 These findings, coupled with the typical appearance of DH lesions on extensor surfaces at sites of trauma, suggest local cytokine/chemokine production after trauma may be one of the inciting factors of DH skin lesions. It may be that after the initial neutrophilic infiltrate binds to the cutaneous IgA, factors such as cytokines, chemokines, and proteases are released that both directly result in blister formation and induce basal keratinocytes to produce collagenases or stromelysin-1 that further contributes to the formation of blisters.28,29 Other studies have suggested that T cells may play a role in the pathogenesis of the skin lesions; however, no specific T-cell responses to gluten have been detected.30,31
It has been known for some time that iodides, administered orally, can exacerbate or elicit eruptions of DH, and this has, in former times, been used for diagnostic purposes. The availability of immunopathologic techniques for the detection of IgA deposits in skin has made such provocation tests obsolete.
The absence of animal models of DH, either naturally occurring or developed in the laboratory, has limited advances in our understanding of the pathogenesis of DH. Recently, Marietta and coworkers reported a new mouse model for DH. They reported an HLA-DQ8 transgenic nonobese diabetic mouse that when immunized with gluten developed neutrophilic skin lesions along with cutaneous deposits of IgA. In addition, withdrawal of dietary gluten resulted in resolution of the skin lesions.32 Further investigation of this mouse model may provide important information regarding the pathogenesis of DH. Finally, there are a few case reports describing the onset of DH with such medication use as interferon, ribavirin, and gonadotropin-releasing hormone analog.33,34
Clinical Findings
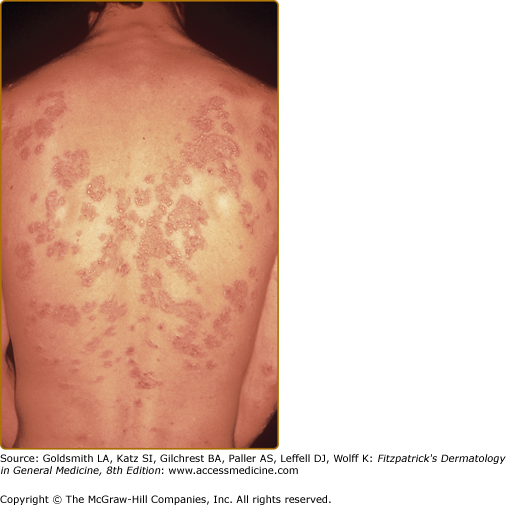
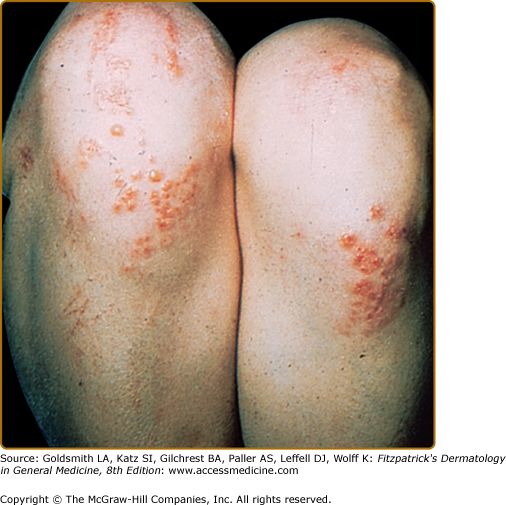
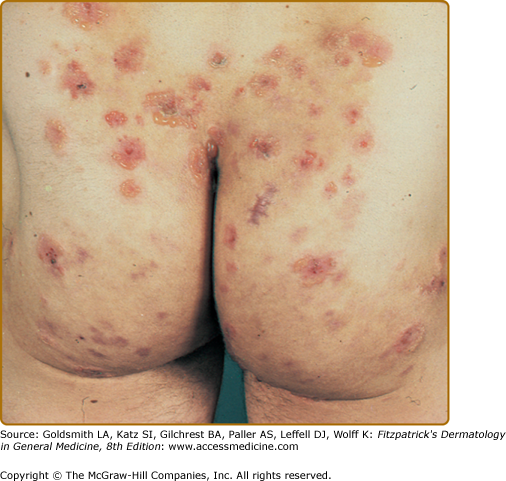
The primary lesion of DH is an erythematous papule, an urticaria-like plaque, or, most commonly, a vesicle (Figs. 61-1, 61-2, and 61-3). Large bullae occur infrequently. Vesicles, especially if they occur on the palms, may be hemorrhagic. The continual appearance and disappearance of lesions may result in hyperpigmentation and hypopigmentation. Patients may present with only crusted lesions, and a thorough search may not reveal a primary lesion. The herpetiform (herpes-like) grouping of lesions is often present in some areas (see Figs. 61-1 and 61-3), but patients also may have many individual nongrouped lesions.
Symptoms vary considerably from the usually severe burning and itching in most patients to the almost complete lack of symptoms in a rare patient. Most patients usually can predict the eruption of a lesion as much as 8–12 hours before its appearance because of localized stinging, burning, or itching.
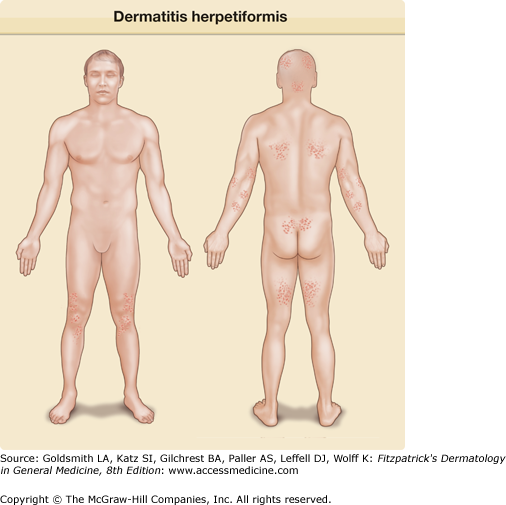
The usual symmetric distribution of lesions on elbows, knees, buttocks, shoulders, and sacral areas is seen in most patients at one time or another (see Figs. 61-1, 61-2, 61-3, and 61-4). Although these regions are affected most commonly, most patients have scalp lesions and/or lesions in the posterior nuchal area. Another commonly affected area is the face and facial hairline. Mucous membrane lesions are uncommon, as are lesions on the palms and soles.
Laboratory Tests
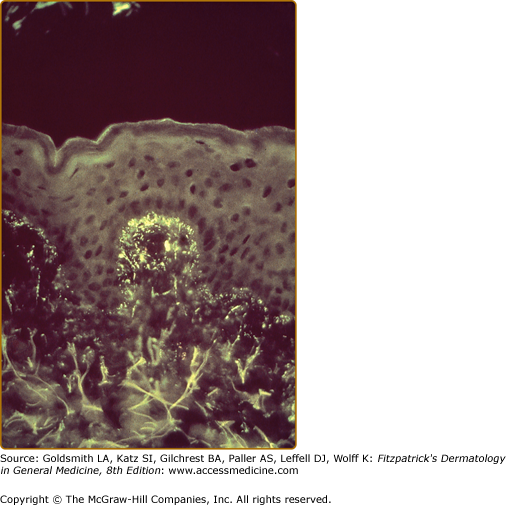
After Cormane demonstrated that both perilesional and uninvolved skin of patients with DH contained granular Ig deposits located in dermal papillary tips, van der Meer found that the most regularly detected Ig class in DH skin was IgA (Fig. 61-5).35,36 Although most patients have granular IgA deposits in their skin, recent studies have suggested that a distinct fibrillar pattern of IgA deposits can be found in some patients with DH.37 The potential clinical significance of this difference is not known. For the most part IgA deposits have not been seen in the skin of patients with isolated GSE (celiac disease).38 Recently, however, Cannistraci and coworkers have used confocal microscopy and reported faint colocalization of IgA and epidermal Tgases in the papillary dermis and in a perivascular distribution in the skin of patients with isolated GSE.39 The significance of these findings in the pathogenesis of DH is not known.
Finding granular IgA deposits in normal-appearing skin is the most reliable criterion for the diagnosis of DH.26,40 These IgA deposits are unaffected by treatment with drugs, but may decrease in intensity or disappear after long-term adherence to a gluten-free diet.41,42 The IgA deposits are not uniformly intense throughout the skin and may be detected more easily in normal-appearing skin near active lesions.43 In DH, other Igs sometimes are bound to the skin in the same areas as the IgA.40 IgA deposits also may be seen in the skin of patients with bullous pemphigoid, scarring pemphigoid, Henoch–Schönlein purpura, and alcoholic liver disease, although in different patterns of distribution than those seen in DH.
Because of the IgA skin deposits and the association between DH and GSE (celiac disease), several groups have studied the IgA subclasses in DH. IgA1 is the predominant (or exclusive) subclass that has been identified in the skin of DH patients.44,45 Most IgA1 is produced in the bone marrow, whereas most IgA2 is produced at mucosal sites. This does not negate the possibility that the IgA1 in skin may still be of mucosal origin because IgA1 is the predominant IgA subclass of IgA antibodies directed against dietary proteins produced in gut secretions in patients with DH. 46,47 Recently, Kantele et al reported a DH association with an increase in circulating IgA1-plasmoblasts with skin-homing receptors (CLA) as compared to those with IgA2.48
The third component of complement (C3) is frequently found in the same location as IgA. The presence of C3 in both perilesional and normal-appearing skin is not affected by treatment with dapsone (diaminodiphenyl sulfone), but C3 may not be detectable after treatment with a gluten-free diet.42,49,50 C5 and components of the alternative complement pathway also may be seen in areas corresponding to the IgA deposits. The C5–C9 membrane attack complex, which is formed as the terminal event in complement activation, is also seen in normal-appearing and perilesional skin of patients.51
The exact site of the IgA deposits in DH skin has been studied by immunoelectron microscopy. Early studies indicated that IgA is preferentially associated with bundles of microfibrils and with anchoring fibrils of the papillary dermis immediately below the basal lamina.52,53 More recent studies, however, have indicated that some or almost all of the IgA deposits are related to nonfibrillar components of skin and other connective tissues.53–55 There is also no agreement as to whether the IgA deposits in DH colocalize to fibrillin, a major component of the elastic microfibrillar bundles.55,56
Antireticulin antibodies of the IgA and IgG classes have been detected in the sera of 17%–93% of patients with DH and in higher percentages of patients with other diseases, especially celiac disease.57 Thyroid microsomal antibodies and antinuclear antibodies also have been detected in increased frequency in the sera of patients with DH.58,59 Putative immune complexes have been detected in the sera of 25%–40% of patients.60,61
Chorzelski et al have described an IgA antibody that binds to an intermyofibril substance (endomysium) of smooth muscle.62 The nature of this antigen has been identified recently by the studies of Sardy et al, who showed that these IgA autoantibodies have specificity for Tgases, particularly epidermis-specific Tgases.21 Although a majority of DH patients on gluten-containing diets have circulating antiepidermal Tgase antibodies, a significant number of patients do not.24,63 Therefore, the presence of circulating antiepidermal Tgase antibodies should probably not be considered as a diagnostic test.
There is a marked increase in the incidence of certain major histocompatibility complex antigens in patients with DH. Worldwide studies have found that 77%–87% of DH patients have HLA-B8 (compared with 20%–30% of unaffected individuals).64–66 In addition, the class II major histocompatibility complex antigens HLA-DR and -DQ are associated with DH even more frequently than is HLA-B8.67,68
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree