Photoprotection: Introduction
|
With increased attention to physical fitness and outdoor recreational activities, daily exposure to sunlight is common. Although sun exposure may have beneficial effects such as mood elevation and vitamin D3 photosynthesis, unwanted effects are well known. Acute effects of sun exposure include sunburn and delayed tanning. Chronic sun exposure is strongly associated with photoaging, actinic keratoses, and squamous cell carcinoma; intermittent sun exposure is associated with basal cell carcinoma and melanoma. Complete avoidance of sun exposure is neither necessary nor practical, nor will it be accepted by the general public. As such, behavioral modification of seeking shade during peak ultraviolet B (UVB) hours of 10:00 am. to 2:00 pm and the use of photoprotective measures, such as sunscreen, clothing, wide-brimmed hat, and sunglasses, and when appropriate, intake of vitamin D supplements, have become the public health message to deliver. This chapter discusses the currently available, commonly used photoprotective measures.1
Sunscreen
The first UVB filter, PABA (para-aminobenzoic acid), was patented in 1943, and the first UVA filter, a benzophenone, was introduced in 1962. In 1972, the US Food and Drug Administration (FDA) reclassified sunscreens from cosmetics to over-the-counter (OTC) drugs, resulting in more stringent regulation. In 1979, long UVA filters, dibenzoylmethane derivatives, became available. In 1990s, the need for protection against UVA, as well as against UVB, was recognized, which led to further development of UVA filters.
The sun protection factor (SPF) was first developed by an Austrian, Franz Greiter, in 1962 and was adopted by the FDA in 1978.2 Current FDA guidelines specifically require that products be tested using a solar simulator with emission spectrum covering the wavelength range of 290–400 nm, and that the sunscreen product be applied at a concentration of 2 mg/cm2.
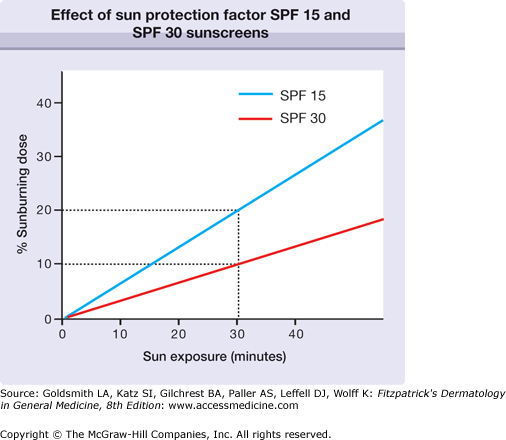
By definition, SPF is the ratio of minimal erythema dose (MED) of a subject’s sunscreen-protected skin over the MED of the unprotected skin. Because the end point is erythema, SPF is a reflection of protection against the biologic effect of UVB (290–320 nm), and to a lesser extent, UVA2 (320–340 nm) (see Chapter 90). The effect of sunscreens with different SPFs on the transmission of erythemogenic rays is shown in Figure 223-1.3 If an individual develops slight erythema after 10 minutes of sun exposure, 30 minutes of unprotected sun exposure result in a 3 MED sunburn. In contrast, when wearing an SPF 15 or SPF 30 sunscreen, the same 30-minute exposure would result in only 20% or 10%, respectively, of an MED. With chronic exposure, the added protection from an SPF 30 sunscreen halves the cumulative UV damage compared to the SPF 15 sunscreen, even though both products prevent sunburn.
Figure 223-1
Effect of sun protection factor (SPF) 15 and SPF 30 sunscreens on an individual who would develop minimal erythema after 10 minutes of unprotected sun exposure. After a 30-minute sun exposure, 20% of a sunburning dose is achieved after the application of SPF 15 sunscreen; after the application of SPF 30 sunscreen, only 10% of a sunburning dose reaches the skin.
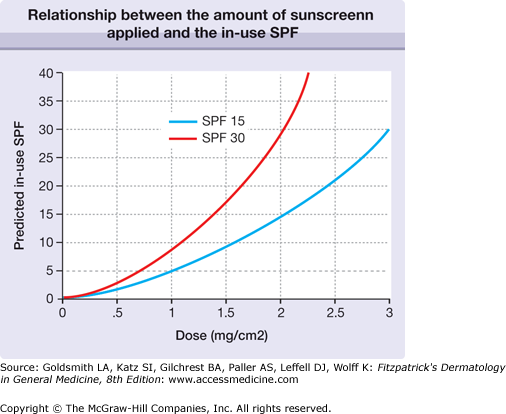
The concentration of sunscreen specified for SPF testing (2 mg/cm2) is the equivalent of 1 oz (30 mL) of sunscreen to cover the entire body surface. Studies of the actual sunscreen usage by individuals have consistently demonstrated that the amount of sunscreen applied is closer to 0.5–0.8 mg/cm2, so that the actual in-use SPF is significantly lower than the label SPF.4 The relationship is not linear, and an SPF 30 product (at 2 mg/cm2) has an SPF of only 3 if applied at 0.5 mg/cm2 (Fig. 223-2).5 This situation causes many users to overestimate their degree of photoprotection and also confounds epidemiologic studies that rely on self-reported sunscreen use to judge the efficacy of sun protection in preventing, for example, skin cancer, including melanoma.
![]() In June, 2011, US Food and Drug Administration (FDA) released the final rule, a component of its Final Sunscreen Monograph.6 FDA mandates that UVA protection of a product be assessed by a pass/fail in vitro test, critical wavelength (λc). Only products with a λc ≥ 370 nm (defined as 90% of area under the absorption curve to be ≥370 nm) are allowed to be labeled as “broad spectrum.” Furthermore, water resistant rating will be labeled as “water resistant (40 min)” or “water resistant (80 min).” Manufacturers have until June 2012 to comply. The decision on capping SPF to 50 has been postponed until a future date.
In June, 2011, US Food and Drug Administration (FDA) released the final rule, a component of its Final Sunscreen Monograph.6 FDA mandates that UVA protection of a product be assessed by a pass/fail in vitro test, critical wavelength (λc). Only products with a λc ≥ 370 nm (defined as 90% of area under the absorption curve to be ≥370 nm) are allowed to be labeled as “broad spectrum.” Furthermore, water resistant rating will be labeled as “water resistant (40 min)” or “water resistant (80 min).” Manufacturers have until June 2012 to comply. The decision on capping SPF to 50 has been postponed until a future date.
SPF is accepted as the worldwide standard for the assessment of protection against the erythemogenic effects of UVB and UVA2. Among the several methods to evaluate protection against UVA, the persistent pigment darkening (PPD) method has become the most widely used in the past few years.5,7 In the PPD method, the dose of UVA required to induce persistent pigment darkening observed 2–24 hours after exposure of sunscreen protected skin is compared to that of sunscreen-unprotected skin; the ratio is then expressed as the UVA protection factor (UVA-PF). In June, 2011, the US FDA decided to use an in vitro critical wavelength test as an assessment of UVA protection of sunscreens sold in the US.8
SPF for a sunscreen product correlates poorly with its ability to protect against immunosuppression. This has resulted in the development of the concept of immune protection factor (IPF), quantifying the ability of sunscreen products to prevent immunosuppression.9 Several different methods have been used, including contact sensitization, and intradermal injection, all of which are quite laborious and time consuming to perform. Until the time that a simple and reliable method can be easily performed in a large number of test subjects, it is unlikely that IPF will be used to rate commercial products.
There are three different nomenclatures used by the sunscreen industry and regulatory agencies around the world: (1) International Nomenclature of Cosmetic Ingredients (INCI), (2) United States Adopted Name (USAN), and (3) trade name. USAN is the nomenclature used in the FDA Sunscreen Monograph. For example, for a widely used UVA1 filter, the INCI name is butyl methoxydibenzoylmethane, the USAN is avobenzone, and a trade name is Parsol 1789. In this chapter, when available, the USAN nomenclature is used.
The term sunblock is commonly used to refer to sunscreens and their active ingredients, but it is a misnomer, and the FDA Sunscreen Monograph does not sanction the term. All sunscreen active ingredients are filters that absorb part of the incident UV radiation, but a portion of the radiation is always transmitted. Microfine inorganic filters additionally reflect and scatter UV radiation.
In the United States, all active sunscreen ingredients are regulated by the FDA as OTC drugs.6 Only UV filters listed in the Sunscreen Monograph issued by the FDA may be marketed in the United States (16 active ingredients; Table 223-1). In addition, filters approved by the FDA as active ingredients of final products through the New Drug Application (NDA) process can also be marketed in the US, as was done for the latest UVA filter available in the US market, ecamsule (Mexoryl SXTM). In 2002, the FDA instituted the Time and Extend Application (TEA) process as an alternate to filing the NDA.10 With the TEA process, for the first time, data generated outside the United States can be used for the application, provided that the sunscreen product has been marketed for OTC purchase for a minimum of 5 years in the country where testing was performed. In the EU, South America, many Asian countries, and Africa, sunscreens are regulated as cosmetics, resulting in a simpler and more expeditious approval process compared to the United States, usually resulting in approval within 1–2 years of filing.
US Adopted Namea | International Nomenclature of Cosmetic Ingredients | λmax (nm); or Absorption Range | Comment |
|---|---|---|---|
Organic filters: UVB | |||
| |||
| PABA | 283 | Stains clothing. Not widely used |
| Ethylhexyl dimethyl PABA | 311 | Most commonly used PABA derivative. Photounstable |
| |||
| Ethylhexyl methoxycinnamate | 311 | Most widely used UVB filter. Photounstable |
| Cinoxate | 289 | — |
| |||
| Ethylhexyl salicylate | 307 | Weak UVB absorbers. Improves photostability of other filters |
| Homosalate | 306 | |
| Triethanolamine salicylate | 260–355 | Weak UVB absorbers. Good substantivity—used in water-resistant sunscreens and hair-care products |
| |||
| Octocrylene | 303 | Photostable. Improves photostability of photolabile filters |
| Phenylbenzimidazole sulfonic acid | 310 | Water soluble. Enhances sun protection factor of the final product |
Organic filters: UVA |