Fig. 13.1
Centrifuged fat tissue for transplantation. Centrifuged fat tissue includes the intact adipocytes, stromal (stem) cells and destructed adipocytes and oil
In this regard, the study intended to identify the components of freshly harvested fat that can clinically affect the volume maintenance rate. We applied different squeezing and condensing methods to remove oils within fat tissues in an attempt to see the increase of stromal cells and substances and the volume maintenance of graft fat. Such a study meant to present a stromal condensation protocol.
13.2 Method
In vitro: Each 50 ml of the aspirated fat from healthy women with age from 20s to 40s were transferred into 60 ml syringes. We divided them into six groups by the different processing method. We removed water and blood, oil naturally separated by gravity for 5 min after harvesting or washing from every syringe (primary removal of impurities) in every group before starting these experiments. For the A group named as a Crude harvested fat, we left the 50 ml lipoaspirates at room temperature for 5 min, induced to be naturally separated into fat, blood fluid and oil portions and we discarded the impurities oil, blood and fluid portions. For the B group named as a Washed Fat, the 50 ml lipoaspirates was washed with DPBS at 1:1 ratio three times and only the fat portion is harvested through removal of naturally separated impurities as above. For the C group as a Simple Centrifugation, we centrifuged at 200 g (1,020 rpm) for 3 min after a primary removal of impurities. For the D group as a Squeezing centrifugation, we centrifuged the 50 ml crude fat tissues in the 50 ml fat processing unit syringe with 100 μm mesh piston (50 ml FPU™, Medikan Co Ltd., S. Korea) at 3,500 rpm (1,924 g) regarding the all different spinning diameter within a long syringe) for 5 min. For the E group as a hand squeezer (Lipo Squeezer™, Medikan Co Ltd., S. Korea), we condensed more with two times squeezing, first with 1.18 mesh piston and then with 0.8 mesh again, and finally centrifuged with a fat processing centrifuge (Lipokit™, Medikan Co Ltd., S. Korea) (Fig. 13.2). For the last F group, a fat mincing micronizer (Filler Geller™, Medikan Co Ltd., S. Korea), we centrifuged first, micronized or minced with a fat mincing micronizer (Filler Geller™, Medikan Co Ltd., S. Korea) at speed level 6 for 9 s and finally centrifuged again. From six groups, we used the equal volume of fat, treated with 0.2 % collagenase, incubated for 30 min at 37 °C, subsequently washed with DPBS three times and finally counted the cell numbers.
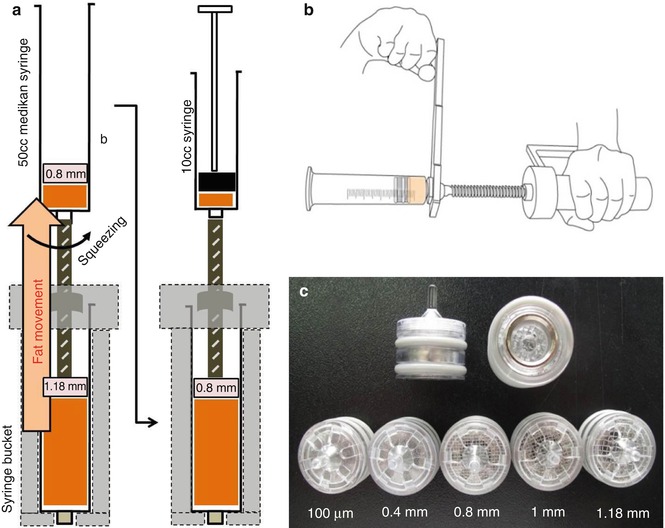

Fig. 13.2
Squeezing process without centrifuge. Fat tissues were squeezed with pistons of 1.18 and 0.8 mm mesh (a), Squeezing technique with Hand squeezer (b), Pistons with different mesh sizes (c)
In vivo: We used an 8th week aged female nude mouse and inject 0.5 ml fat from each 6 group into both its hypoderms with 21G needle. We took fat graft at 4th and 8th weeks respectively, measured each weight and specific gravity and finally analyzed the physiological structures of graft tissue using Masson Trichrome (M.T.) dyeing method.
13.3 Results
13.3.1 Fat Separation and Measuring Cell Numbers Within SVFs
From groups like Crude harvested (A), Washed (B), Simple centrifuged (C), almost no oils were separated. On the other hand, from the groups like Squeezing centrifugation (D), Hand squeezing (E), Minced micronizing (Filler Geller™, Medikan Co Ltd., S. Korea) (F), oil and fat were clearly separated by different layers (Fig. 13.3). The cell numbers were highest in group E and F with no significant difference and lowest in group A with naturally induced separation. The result shows us the more we removed oil and water, the more we got the number of ASCs from the fat. It means the removal rate of oil and water is directly related with the increasing rate of ASC. We verify the quality of the transplanted fat from the simple formula for SCR (Figs. 13.3 and 13.4).
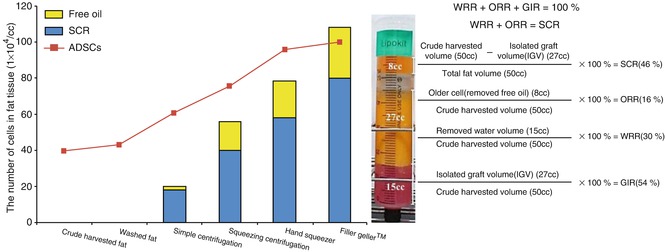
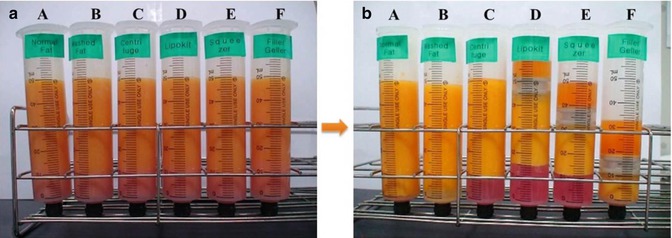

Fig. 13.3
Fat in accordance with the ratio of oil removal and the amount of SCR reaching high. The amount of oil removal and the proportion of the cell concentration subsequently went up. Crude harvested volume total fat tissue volume after a primary removal of impurities separated by gravity for 5 min, SCR stromal condensation rate after squeezing processes, a proportion of reduced volume through squeezing centrifugation, which indicates the proportion of cell density increase, 46 %, ORR oil removal rate after squeezing processes, indicate the proportion of destructed adipocytes, proportional to the amount of older cells, 16 %, WRR water removal rate after squeezing processes, 30 %, GIR graft isolation rate after squeezing processes, 54 %

Fig. 13.4
Separation of human adipose tissue. Before processing, primary removal of impurities were done (a), after processing of their own. Group A, B; no condensation. Group C; low SCR (stromal condensation rate). Group D; moderate SCR. Group E; high SCR. Group F; the highest SCR (b)
Primary removals of impurities were done in all groups. Those mean manual removals of water, free oil, blood components from standing syringes at 5 min points at room temperature after harvesting.
Group A was naturally induced for separation for 5 min once more (Crude harvested group).
Group B was washed with DPBS three times (Washing group).
Groups C was centrifuged at 200 g (1,020 rpm) for 3 min (Simple centrifugation group)
Group D was centrifuged with a fat processing centrifuge (Lipokit™ & 50 ml FPUTM, Medikan Co Ltd., S. Korea) at 3,500 rpm for 5 min (Squeezing centrifugation group).
Group E was manually squeezed by 1.18 mm and 0.8 mm mesh piston and centrifuged 200 g (1,020 rpm) 3 min, then secondary removal of impurities, then centrifuged with a fat processing centrifuge at 3,500 rpm (1,924 g) for 5 min (Hand squeezing and squeezing centrifugation group).
Group F was centrifuged with a fat processing centrifuge at 3,500 rpm (1,924 g) 5 min, then secondary removal of impurities, then minced micronized with a fat mincing micronizer (Filler Geller™, Medikan Co, Ltd., S. Korea) at a speed level 6 (15,000 RPM of cutter) for 9 s (Mincing and squeezing centrifugation group).
Group A, B; no condensation,
Group C; low SCR (stromal condensation rate).
Group D; moderate SCR
Group E; high SCR
Group F; the highest SCR
SCR and free oil removal rates were high in Groups, D, E and F and also ASCs (adipose-derived stem cell) numbers were high in those groups (Fig. 13.5a).
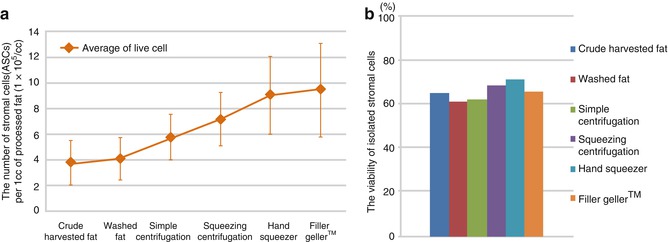

Fig. 13.5
ASCs numbers, stromal condensation rates, released oil volume by each 6 group. ASCs numbers per 1 ml (a), Cell viability by percentage (b)
Cell numbers per 1 ml and cell viabilities in each group (Fig. 13.5b). Collagen of adipose tissue was digested within a vertical blending incubator (Maxstem™, Medikan Co Ltd. S. Korea). After rinsing with distilled water, cell counts were performed (n = 4).
13.3.2 Fat Graft and Volume Maintenance Check-up
Blood vessels were formed well around the grafted fat and fat cells were kept well in a globular shape (Fig. 13.6).
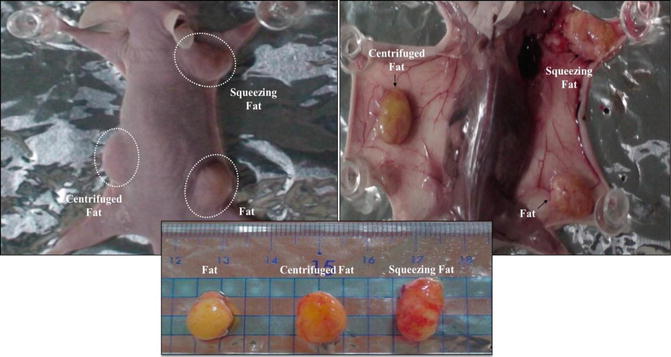

Fig. 13.6
Well circumscribed subcutaneous lump at the backs of nude mice. The fat graft was obtained at 4th and 8th week
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








