Fig. 22.1
Manual liposuction with a 10-mL syringe from the abdomen

Fig. 22.2
Centrifugation of lipoaspirates
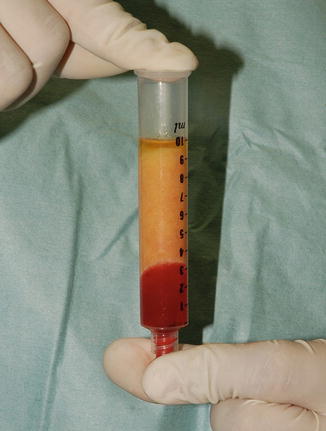
Fig. 22.3
Three layers of the lipoaspirate after centrifugation. Only the middle layer is injected
22.4 Composition of Fat
In the past, fat tissue was regarded as a homogenous mass with energy storage being its primary function, but research revealed the diversity of components resembling this dynamic and homeostatically relevant organ [10]. Collagenase digestion divides fat into two fractions: a layer of mature adipocytes floating on the surface and a layer consisting of all remaining cells composing the stromal vascular fraction (SVF). The SVF includes multiple cell lines, for example, fibroblasts, erythrocytes, endothelial cells, myocytes, leukocytes and adipose-derived stem cells, which exhibit a pivotal role in tissue regeneration [11].
22.5 Adipocyte-Derived Stem Cells
Although today’s experts mostly focus on the mesenchymal stem cells, the significance of adipocytes in fat should not be ignored. Mature adipocytes of course do not possess the same properties as mesenchymal stem cells but still regulate cell survival, angiogenesis or inflammation and secrete over 100 cytokines [12–14].
Nevertheless, the main interest lies in adipocyte-derived stem cells, and the differentiation into adipogenic, osteogenic, chondrogenic, myogenic and importantly also haematopoietic cell lineages was demonstrated in various publications [15]. They possess high proliferation rates with low oxygen consumption and release numerous proangiogenic growth factors, on the one hand, and differentiate into endothelial cells, on the other hand, to ultimately promote blood vessel formation. Since the proangiogenic potential of lipografts, above all the plasticity of ASCs, was discovered, scientists speculate about their benefits in different fields of medicine, e.g. as a legitimate alternative to revitalise ischaemic tissue [16]. As illustrated in animal burn models, fat grafts accelerate neovascularisation after burn injury, which in turn leads to a downregulation of pathways responsible for tissue fibrosis [17]. Furthermore, the local application of ASCs improves skin quality and dermis thickening by the neosynthesis of collagen fibres. In an experimental animal study, Mojallal et al. showed an increased collagen-1 production of mouse fibroblasts stimulated by injected human fat. The authors hypothesised that fat grafts represent a natural filler with volumetric and regenerative effect. The regenerative effect consists of dermis thickening through collagen-1 production, improvement of skin texture and elasticity and colour modification through diminishing local inflammation. These effects are attributed to the differentiation of ASCs and release of growth factors [18, 19].
22.6 Growth Factors
Adipokines are cytokines which are secreted by fat tissue including growth factors which promote cell growth and differentiation. During lipofilling procedures, not only cells with a volumetric effect but also growth factors and growth factor-producing cells are transferred.
The knowledge about growth factors expands progressively and many scientists focus on the regenerative effect of these cytokines on tissue. In ageing skin, the amount of growth factors and growth factor-producing cells decreases continuously. Nowadays a well-balanced mixture rather than single growth factors is regarded to be responsible for tissue regeneration [20]. Thus, the natural mixture and concentration of growth factors of fat grafts, resembling a physiological body state, are a reasonable and innovative approach in skin rejuvenation. The regenerative impact of growth factors has been investigated in a variety of clinical trials, mostly using topical cytokine-enriched creams. The rejuvenating effect of growth factors in combination with hyaluronic acid and a micro laser peel was described by Gold et al. [21]. Omi et al. [22] chose a combination of growth factors, pneumatic energy and broadband light and showed collagen and elastin fibre formation in histological analysis. Many more articles provide evidence for the rejuvenating effect of growth factors [23–25].
Aside from tissue rejuvenation, the promotion of fat graft vascularisation is another important aspect of growth factors since the vascularisation of the graft on the recipient site poses a major challenge. Neoangiogenesis, the formation of new blood vessels, is crucial due to the fact that nutritive supply of cells by diffusion is only possible in the range of a few millimetres. Therefore, until neoangiogenesis and inosculation take place, the fat transplant should be imbibited. Especially in case of high volume transfers, the vascular system of the recipient site might be insufficient leading to fat necrosis and resorption.
The vascular endothelial growth factor (VEGF) is one of the growth factors and plays a key role as a proangiogenetic factor promoting angiogenesis and neoangiogenesis. Differentiation, tubulogenesis, migration, survival and proliferation of vascular endothelial cells, regulation of vascular permeability are all mediated by VEGF. In addition, the plasminogen activation and matrix metalloproteinase system are orchestrated by this soluble factor [26]. The importance of VEGF is underlined by the fact that a loss of a single VEGF-allele or deficiency of its receptor VEGF-R1 or VEGF-R2 is lethal in mice. Alongside VEGF there are many more proangiogenic growth factors such as the hepatocyte growth factor (HGF), basic fibroblast growth factor (bFGF), granulocyte-macrophage colony stimulating factor (GM-CSF), transforming growth factor-β (TGF- β), insulin-like growth factor (IGF-1), platelet-derived growth factor (PDGF-BB), adiponectin, stromal-derived factor 1 (SDF-1), tumour necrosis factor-α (TNF-α) and leptin [27–29]. Each angiogenic growth factor has a distinct function within a delicately regulated system, which has not been fully explored yet. For instance, the basic fibroblast growth factor supports blood supply by enhancement of endothelial cell migration and proliferation [30].
The application of single growth factors did not show an entirely convincing result in fat graft vascularisation since a complex mechanism with synergistic effects of a multitude of factors is likely. Lipofilling facilitates the engraftment of ASCs, which provide a regulated growth factor secretion and can actively respond to hypoxia [27, 31–33]. In fact, a paracrine amplification mechanism for HGF and VEGF was reported early [34]. However, the content of basic fibroblast growth factor, insulin-like growth factor (IGF-1), vascular endothelial growth factor and platelet-derived growth factor (PDGF-BB) in lipoaspirates was assessed in a study performed in our workgroup showing high levels of the abovementioned growth factors in the native lipoaspirate, highest concentrations in the purified fat and biological activity even after storage of lipoaspirates [28].
The pretreatment and simultaneous injection of growth factors are methods to enhance fat graft survival. Although the theory of molecules with vascularising property improving graft survival appears sound at first, experimental studies show controversial results [35].
22.7 Growth Factors and Bioengineering
The modern field of bioengineering deals with the establishment of appropriate scaffolds loaded with adipocytes, ASCs or growth factors securing a higher graft survival. Biodegradable carriers, such as meshes, gels or microspheres made of collagen or hyaluronic acid, gelatine or other materials, have been tested with no consensus about an optimal three-dimensional scaffold providing adequate cell survival and proliferation properties found so far [36, 37]. In ageing skin, the amount of growth factors and growth factor-producing cells decreases continuously.
In animal studies, VEGF-transfected ASCs lead to an improvement of fat graft survival [38]. New approaches in the field of tissue engineering try to load lipoaspirates with VEGF encapsuled in microspheres which protect VEGF from denaturation to increase graft survival and improve vascularisation [39]. If experimental protocols can be optimised, these methods are promising options to allow appropriate perfusion of even bigger fat transplants.
Lu et al. [38] recently reported about genetically modified ASCs which improved fat graft survival. VEGF was transduced in ASCs and injected subcutaneously in mice and histologically showed a higher density of capillaries. Beside the survival of the fat graft itself, proangiogenic factors such as VEGF improve vascularisation on the recipient site by stimulation of endothelial differentiation and migration.
A completely different approach to improve the vascularisation of fat grafts is the blood supply of bioengineered cell-seeded chambers via pedicles. Even though animal experiments did show reliable results, this technology has not been fully developed to date [40].
22.8 Clinical Application
The numerous benefits of fat allow vast clinical application for the correction of volume deficits of congenital, traumatic, postsurgical or age-related nature and, additionally, the treatment of skin texture, colour and quality.
Reconstruction of bony lesions due to the potential of ASCs to promote bone formation was reported in different publications. The reconstruction of a calvarial defect after severe trauma in a 7-year-old girl with autologous fat is one of the examples [41]. Parry-Romberg’s syndrome, a rare and slowly progressing hemifacial atrophy starting with skin, subcutaneous, fat and muscle degeneration and ending in deterioration of bone and cartilage, was successfully treated with autologous fat [42–44]. Besides the beneficial properties as a sheer filler, the potential of ASCs to promote angiogenesis and differentiate into each of the deteriorated cell type makes fat transplants a prime candidate for the reconstruction of this disease. In contrast to fat graft alone, the simultaneous injection of fat and isolated and cultured ASCs leads to a significant enhancement of fat graft survival [44].
Particularly the correction of scars governed by ASCs and growth factors within the lipograft is an invaluable and unique potential amongst fillers. Despite continuous scientific progress, scars remain a wearing issue and an aesthetic, functional and psychological burden for patients. Lipofilling of scar tissue is a new surgical tool which improves texture, colour and even the compliance by modulating the scar collagen and revascularising scar tissue [45]. Scars after radiation therapy or acne represent further therapeutic fields of lipofilling [46]. Of course, many patients present with aesthetic indications and ask for treatment of for instance age-related contour deformities. In Fig. 22.4 a young female patient with hollow eyelids was treated with autologous fat grafts harvested from her abdomen.
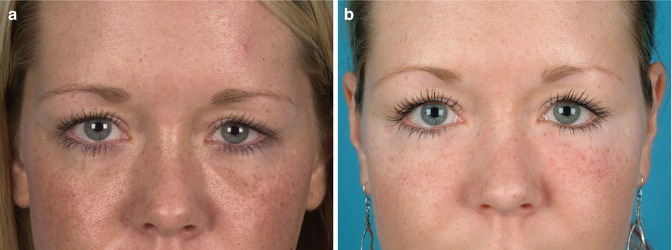

Fig. 22.4
(a) Preoperative patient with hollow eyelids. (b) Postoperative following autologous lipofilling









