Fig. 40.1
VASER system (by Sound Surgical Technology, Denver, CO, USA)
At the beginning, this was only used for body-contouring purposes and then for breast reduction, neck-chin debulking and contouring, circumferential thigh liposuction, and lastly axillary hyperhidrosis treatment, just to mention a few of the potential applications of this sophisticated device. Recently, the application of VASER™ has been used for fat harvesting and autologous fat transfer (AFT) for breast enlargement and contouring and adipose-derived stem cell treatments [1]. The technique presents fewer side effects long term in comparison with traditional implants and is safe and effective if properly performed.
Traditionally, physicians have used handheld syringes, following the Coleman technique. This method is both laborious and time consuming and, depending upon the size of the needle and syringe used, may still disrupt fat cells. Performing bilateral breast augmentation AFT requires large amounts of harvest material (minimum 500 mL unfiltered material). Over the last 3 years, standard VASER™ treatment has been used, reducing the power to 60 % of the total at continuous setting, followed by low-vacuum aspiration using the VentX System, and achieved a raw fat viability of 83 %. This high level of viability did not come at the expense of operative time or convenience. An average operating time of 3½ h is required, including using VASER™ to two to four areas for lipoplasty, fat harvesting, PureGraft® washing and preparation, and fat introduction into both breasts with small syringes.
All treatments were performed under tumescent anesthesia and intravenous (IV) sedation. All patients were treated as day case with discharge few hours after surgery. A series of 120 cases, with regular follow-up to 3 years, have been performed.
An important factor in the viability is the use of lower-power VASER™ to emulsify the adipose tissue before aspiration. Separating the adipose tissue into smaller clumps in situ permits the use of atraumatic cannulas and lower vacuum levels, which, in turn, avoids traumatic injury to the fat during the aspiration. Also, by maintaining the overall cellular distribution and ratio of the lipoaspirate (mature adipocytes and the “loosely adherent” cell population (peri-adipocytes, stem cells, stromal cells, etc.)), the retention rate of the implanted tissue matrix is enhanced. In addition to the use of harvested fat for immediate reinjection, adipose tissue is a significant source of stem cells for both the enhancement of autologous fat grafts and for the use in often developing clinical treatments.
As with direct autologous fat injection, the viability of the regenerative cells derived from the harvested adipose tissue is of primary importance, both as fresh tissue and as tissue after freezing. The field of clinical treatments using adult stem cells continues to expand, and patients undergoing liposuction are increasingly interested in storing tissue samples containing stem cells. The tissue could then be thawed at some future date and utilized in the development of genetic and regenerative treatments. The viability of the adipose-derived stem cells was higher than that for the overall tissue samples at over 87 % (Fig. 40.2). Even more importantly, the viability of the adipose-derived stem cells was only slightly reduced to 75 % after freezing using liquid nitrogen vapor. In this application, the harvesting of the adipose tissue using the VASER™ system minimizes tissue damage and improves the viability of the harvested cells.
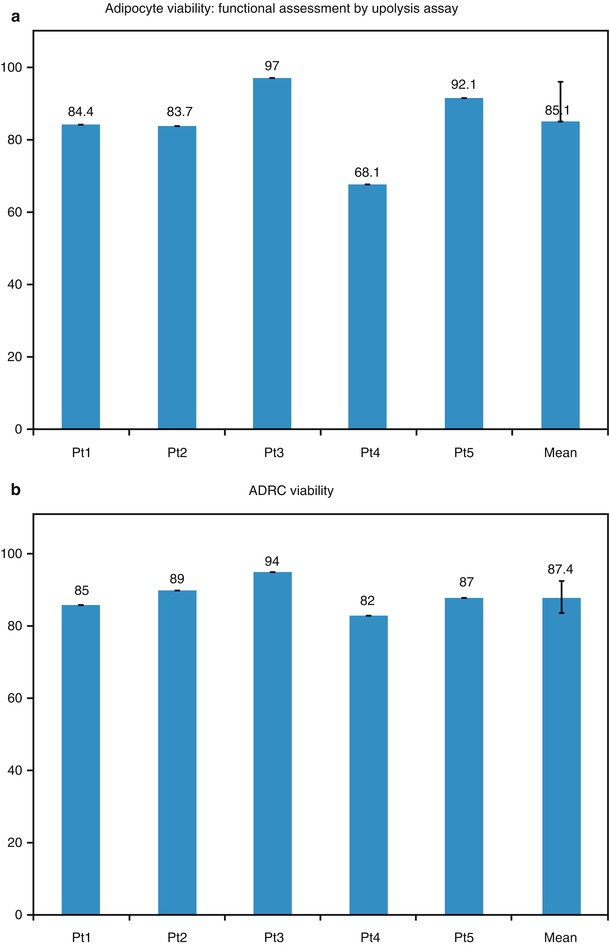

Fig. 40.2
(a) Adipocyte viability: functional assessment by lipolysis assay. (b) ADRC viability
40.2 Patient Selection
All the patients selected for bilateral breast augmentation to improve shape, contour, and volume using VASER™ as primary source of fat harvest followed these criteria:
1.
No significant PMH or strong family history of breast cancer.
2.
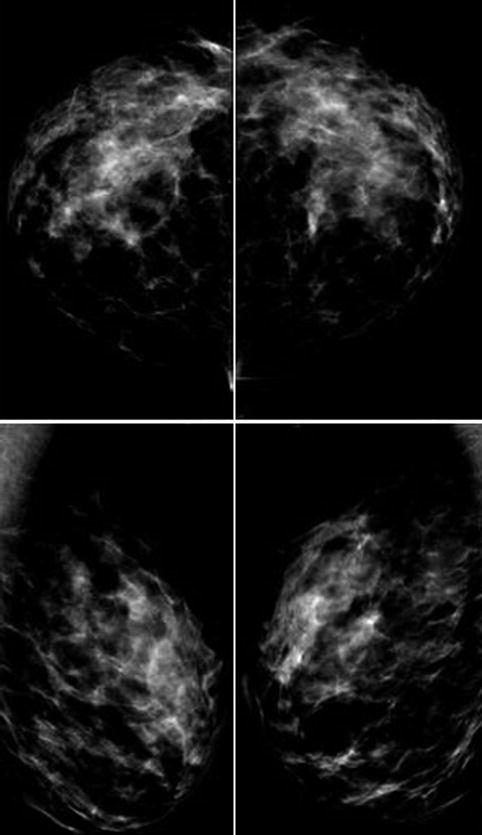
Mammography: Preoperative as baseline to exclude pathology. Patients with fibroadenoma, suspected cysts >1 cm, or significant calcifications are discouraged.
3.
Patients with moderate to severe glandular or skin ptosis are denied surgery (Fig. 40.3).
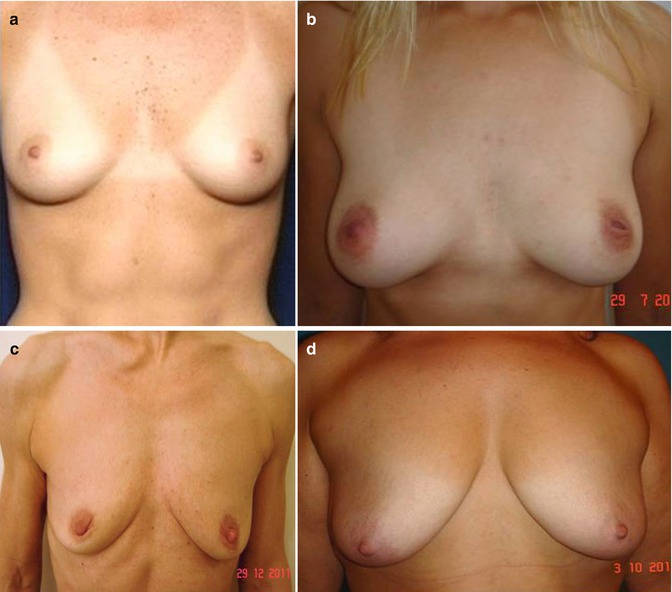

Fig. 40.3
(a) Good candidate for fat transfer (good skin tone, elasticity, nipple position). (b) Good candidate for fat transfer (mild ptosis). (c) Poor candidate (breast ptosis, poor skin tone, no projection). (d) Contraindication to fat transfer for breast enlargement (severe breast ptosis)
4.
Fat donor area (Fig. 40.4). Extremely slender patients are denied surgery (Fig. 40.5).
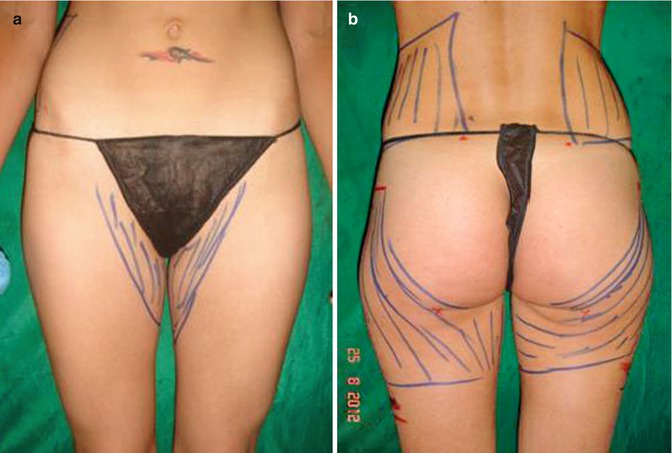

Fig. 40.4
(a, b) Preoperative donor area for fat transfer: trochanters, inner thighs, and flanks. From this patient, a total of 1,800 mL emulsion was removed and 600 mL of PureGraft was transferred

Fig. 40.5
Extremely slender patient. Not an indication for fat transfer
40.3 Patient Assessment
All candidates underwent:
1.
Preoperative clinical evaluation.
2.
Photographs.
3.
4.
Some patients had 3D imaging pre- and postoperatively to assess fat survival and fat distribution in long-term follow-up.
5.
In preparation for surgery, standard ECG and blood test.
6.
All patients operated on were in the ASA 1–2 categories.
7.
Patients were warned to maintain their weight in the postoperative period.
8.
Weight loss could severely influence fat retention. Hormonal changes, as in pregnancy and menopause, may affect fat retention, decreasing the breast volume.
9.
Patients with unrealistic expectations of large volume were denied surgery.
40.4 Selection of Site of Fat Harvesting (Fig. 40.8)
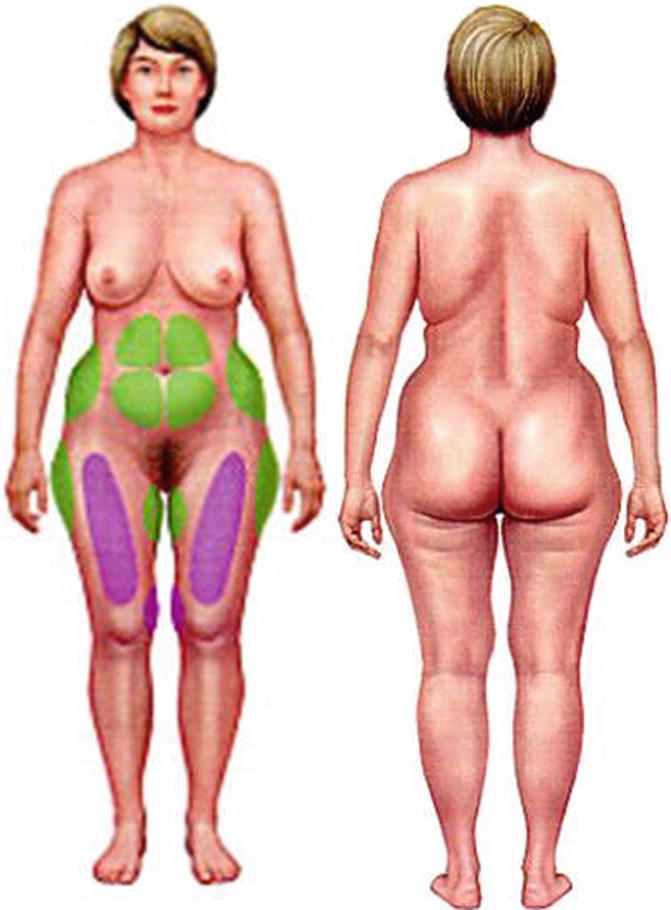
Fig. 40.8
Sites for fat harvesting
1.
Abdomen
2.
Flanks
3.
Anterior thighs
4.
Outer thighs
5.
Arms
6.
Buttocks
40.5 Selection of Anesthesia
All the patients in this series were operated on in an office-based surgery, with local tumescent anesthesia and IV sedation [2].
Tumescent anesthesia consisted of:
1.
1,000 mL normal saline (0.9 %)
2.
1 mL adrenaline (1:1,000)
3.
500 mg lidocaine
The author (DW) uses 600–800 mg of lidocaine routinely in the tumescent anesthesia. Intravenous sedation is given by an anesthetist, on a fully monitored patient, consisting mainly of propofol, fentanyl, midazolam, and IV paracetamol. Sedation was mainly light in the majority of patients remaining conscious and conversant. Fentanyl and/or Fentanest was added, following patients’ analgesia requirement, according to anesthetist protocol and indications. Breast tumescence infiltration ranges from 100 to 300 mL (Fig. 40.9).
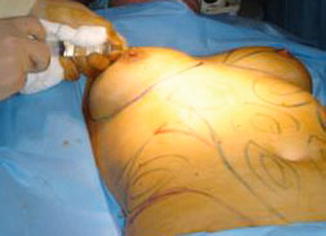

Fig. 40.9
Local anesthetic in periareolar incision
Harvest site infiltration ranges from 2,000 to 4,000 mL. Aspirate ranges from 800 to 3,000 mL.
PureGraft ranges from 280 to 400 mL. All patients had intraoperative antibiotics.
Author, Di Giuseppe, uses Metronizadole IV (400 mg) and Cephotaxime (1 g) followed by 5 days of oral course of the same antibiotics, and antiemetic drugs and gastroprotectors were given routinely. Author, Wolf uses Coamoxiclav 1.2 g IV 5 days oral postoperatively.
All patients were discharged 1–2 h on average after surgery. Typical dressing includes compression garment (apart from breast, where a light support bra (impact level III) or brassiere is advised). Absorbable pads are used for postoperative leakage. Soft silicone drains (corrugated) are positioned at the lower abdomen. Postoperative care includes analgesics (if needed); on the first and second days, postoperative follow-up is done by the surgeon. Manual lymphatic drainage (MLD) starts on the third day postoperative, followed by LPG (Endermologie or similar) or VelaSmooth program post-liposuction, for 6–8 weeks on areas treated.
Breasts need no compression for the first 3–4 weeks (i.e., sleeping in supine position). Swelling normally lasts about 3 weeks. 2-pillow elevation of the thorax for the first 2–3 weeks is advised to reduce swelling.
Light bra is worn for a few weeks.
Postoperative photos are taken at 3, 6, and 12 months after surgery. None of the patients in this series have had postoperative infections.
40.6 Markings (Fig. 40.10)
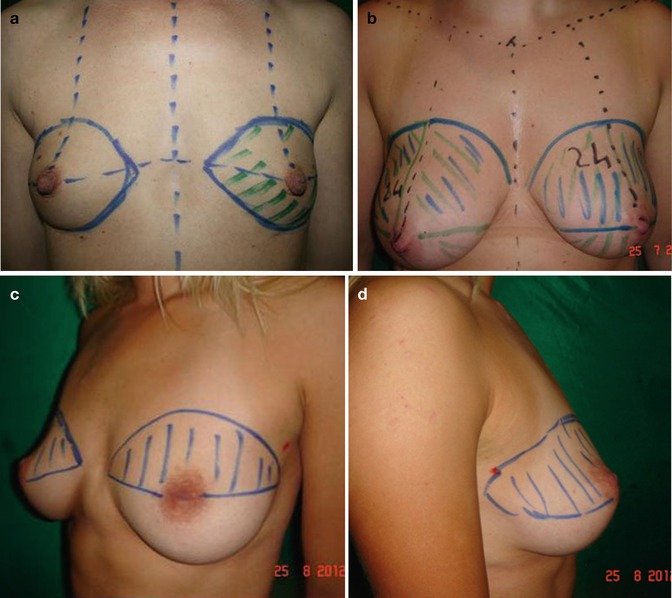
Fig. 40.10
Markings are different depending on breast size and area to be filled. The breast is normally divided into four quadrants. (a) Augmentation in small breast. (b) Augmentation and breast ptosis correction. (c) Post-removal of breast implants. (d) Upper quadrant volume deficiency; lack of fullness in the upper lateral area
Markings are done in standing position with arms down. Preoperative markings and drawings are similar to the standard breast augmentation care:
1.
Tridimensional point is marked bilaterally.
2.
Nipple alveolar complex distance is marked bilaterally with distance from clavicle and clavicular notch.
3.
Inframammary crease is marked bilaterally.
4.
A straight line is drawn from clavicular notch to umbilicus.
5.
The four breast quadrants are marked.
6.
Nipple dimension and areola dimensions are marked.
7.
Asymmetries of position of nipple and of the areola and breast volume are determined.
8.
Consistency of breast tissue is noted.
9.
Areas of volume deficiencies are marked.
10.
The outer margins of the volume-deficient areas are marked, similarly to the standard breast augmentation, where the limits of pockets undermining before implants placement are marked.
40.7 Incisions for Infiltration
The following incisions for infiltration are marked in each breast (Fig. 40.11):
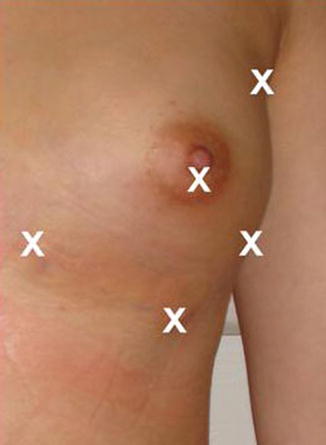





Fig. 40.11
Incision sites
1.
Periareolar
2.
Axillary
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree