The Highly Cohesive, Style 410 Form-Stable Gel Implant for Primary Breast Augmentation
Bradley P. Bengtson
The Natrelle 410 highly cohesive, form-stable gel breast Implant was developed in the early 1990s in conjunction with McGhan Medical Corporation as part of the search for a more optimal filler for a shaped breast implant (1) (also, J. B. Tebbetts, private communication). It was first released in Europe in 1993, secondary to the Food and Drug Administration (FDA) moratorium placed on silicone gel devices in the United States in 1992 and has been described as a fifth-generation device (2). This delay has placed plastic surgeons in the United States at a distinct disadvantage: Even though we have been the innovators in conjunction with industry and developed these devices, we have been unable to use them clinically in an unrestricted manner. In February 2001, a premarket approval study monitored by the FDA was initiated at approximately 80 sites in the United States by surgeons with shaped-implant experience. To date, more than 12,000 patients have been enrolled, and as of this writing, we are beginning the ninth year of enrollment. This chapter discusses the specific characteristics of the Style 410 device, its disadvantages and advantages, tissue-based planning principles specific to it, patient and implant selection, differences in surgical techniques when using this device, personal, national, and global experience along with published results with the 410, and some pearls of wisdom and caveats when using this device.
Characteristics
Form Stability
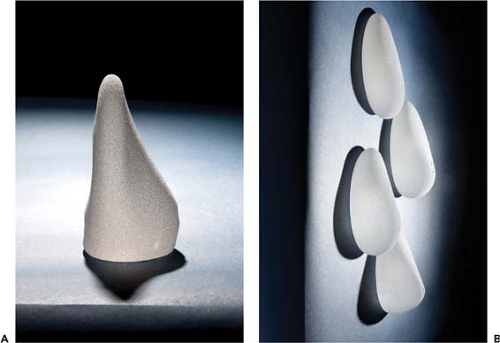
Form stability may be defined clinically as the ability of a device to hold its shape in all positions. In the upright position such an implant is unique in that it has no collapse or bending of the upper pole whatsoever (Fig. 116.1). Secondarily, it has no inherent wrinkling or rippling when held in various positions and maintains shape retention and memory. In the absence of any capsular contracture forces, the implant itself defines the shape of the breast, in contrast to a standard gel implant, for which the breast defines the shape of the implant.
Cohesivity
Form stability is slightly different than cohesivity. Form stability describes the device as a whole—the shell and filler characteristics—whereas cohesivity describes the physical characteristics of the internal gel filler. Silicone gel breast implants have various degrees of internal gel cohesivity. Cohesivity is measured as a stiffness coefficient, which has somewhat of a negative connotation, but in fact measures the degree of cohesivity of the filler. The 410 has the greatest degree of cohesivity of any implant. There is a softer, less cohesive filler called the Soft Touch, which is similar in stiffness/cohesivity to the Mentor CPG device. These two devices are in turn more cohesive than the standard Responsive gel or MemoryGel devices on the market. This increase in cohesivity also provides a less fluid filler and less likelihood of gel to exude from the shell if it is cut or damaged (Fig. 116.2). This increase in cohesivity does create a slightly increased degree of firmness in the hand at room temperature; however, there is a common misconception among patients and surgeons that the 410 is overly firm following implantation. This is in fact not the case. The 410 creates a “one-breast feel” after augmentation and will be further explored in this chapter (3) (Fig. 116.3).
Biocell Texturing
The 410 has an extremely coarse, heavily textured surface of irregular pores in the range of 300 μm (Fig. 116.4). This texturing facilitates holding the implant in position along with a tight hand-in-a-glove pocket dissection discussed later. This texturing and the 410’s form stability are likely contributing factors to its low reported capsular contracture, rotation, and malposition rates (4,5,6).
Matrix of Options
There are approximately 200 different shapes of the 410 in 12 separate cells varying in height, projection, and width (Fig. 116.5). These options provide a wide and unique range of implant selections that can be dialed in to accommodate any patient’s measurements and breast tissue characteristics. These devices are described first by their height— full (F), moderate (M), and low (L)—then by their projection—extra (X), full (F), moderate (M), and low (L)—and finally by their base width in 0.5-cm increments. The available of these options, however, represents a bit of a double-edged sword: The great thing about the 410 is there are so many options available, but the challenge of the 410 is that there are so many options available! There is a method in the works to help simplify implant selection with the 410, and this overall process will be described in detail.
Advantages and Disadvantages
These myriad unique factors also create a number of advantages and disadvantages or trade-offs when considering using the 410 device. Although there is not one implant that is best
for every patient or in every circumstance, the 410 certainly increases the palate of options for the breast augmentation patient.
for every patient or in every circumstance, the 410 certainly increases the palate of options for the breast augmentation patient.
General Disadvantages include the following:
The implant is more expensive initially (but early results increased longevity at 9 years) (7).
It has the potential to rotate, being a shaped device (full height in particular).
The implant is more technically demanding to use surgically.
The implant is more difficult to use in secondary or revision cases, requires a new or virgin pocket for placement, and does not fill the envelope like a responsive gel or low-cohesive filler.
It creates less of an ideal result in patients with loose skin and inability to fill the breast envelope.
There is some increase in edge palpability secondary to form stability and cohesivity.
Implant selection is more challenging.
Tissue-based planning principles are an absolute.
Its use requires a new mindset and should be considered as a new technique (further discussed in Precise Surgical Technique section).
General Advantages include the following:
The implant provides form stability with no inherent wrinkling, rippling, or implant collapse.
It comes in a wide variety of shapes, including different widths, heights, and projections (410 Matrix), providing a huge range of options.
It is less likely to “leak” with a more highly cohesive filler.
The Biocell surface encourages adherence and less mobility with softness.
The implant performs well in challenging, thin patients with less soft tissue coverage and in particular constricted breasts.
It has the ability to “shape the breast” versus having the “breast shape the implant.”
The 410 has the lowest capsular contracture rates reported in the literature.
There is very low visible and palpable wrinkling and rippling.
Lower shell failure rates have been reported with the 410 versus standard gel devices,
In addition, Heden lists some of the specific additional advantages of the 410 implant’s form stability (7,8):
It imparts shape to the breast.
It resists deforming forces from a capsular contraction.
It resists implant collapse and has no inherent wrinkling or rippling or implant irregularities.
It limits gel content motion and may increase implant longevity by decreasing wear on the envelope.
It allows for easier explantation with minimal to no gel extravasations and no implant changes even after many years, except for occasional yellow-serous discoloration.
It may be a major advantage particularly in breast asymmetry cases and in constricted breasts as well as in breast reconstruction and patients with thoracic or chest wall deformity.
The disadvantages of form stability include the following:
It imparts a slightly firmer consistency to the breast.
It decreases deformation, and thus it is difficult to insert through a small incision; a larger incision is required or the gel may fracture (5.5 cm or greater).
Incision is not limited to, but is preferential to, the inframammary fold (IMF).
Tissue-Based Planning Principles
In the past, breast augmentation was viewed as a procedure or technical exercise. However, more recently this procedure has been more properly recognized as a series of processes that are important for a consistent, quality results. These biodimensional principles have been redefined and expanded into a complete process of breast augmentation (9). This process includes patient education/informed consent, tissue-based preoperative planning, refined surgical technique, fast-track recovery, and defined postoperative care.
One of the first critical aspects when using this implant is that the 410 requires a transition from a purely volumetric method to a defined, measurable, planned, dimension-based approach for breast augmentation to include specific patient measurements and breast soft tissue characteristics. A much more detailed planning system and patient evaluation is also required when using this device when adding a third dimension of height into the equation. Because the use of the 410 is much less forgiving, if prior subjective, volume-only evaluation techniques are applied to the use of this implant, results will be less than optimal and lead to higher complication rates and dissatisfied patients. Stated another way, if tissue-based planning principles and processes are not an integral part of the planning, I would recommend not using this device.
Style 410 Patient Selection and Education
There is not one best implant for every patient. Each implant has advantages and disadvantages, or trade-offs, that must be weighed and discussed with each patient preoperatively. Figure 116.6 gives a basic algorithm with general guidelines for patient selection. Patients encouraged to select a standard gel device include, first and foremost, patients with a loose skin envelope where the breast dimensions cannot be filled. This is the main patient dissuaded from the 410: the patient with an envelope-to-implant mismatch. Also in this category are patients with significant ptosis where a vertical to full mastopexy or lifting the nipple more than 4 cm above the transposed inframammary fold is called for. In addition, in patients with significant chest wall deformities such as pectus carinatum or excavatum, I prefer a standard gel device that blends into the chest wall defect to a greater degree. When a form-stable device is used in these types of patients the implant becomes an extension of the chest, and instead of molding into the rib/sternal deformities, it tends to accentuate them. Mild to moderate asymmetry may be improved with this device, but marked breast asymmetry greater than 150 to 200 cc is difficult to judge. Style 410 sizers and in particular three-dimensional imaging and simulation are creating major strides in improving more predictable outcomes with the 410 in patients with significant asymmetry. Although this chapter deals with primary augmentation, unless one is revising a small subglandular augmentation with a partial submuscular device or performing a neo-subpectoral pocket
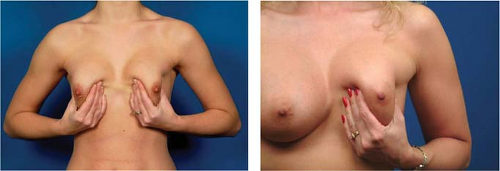
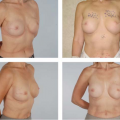
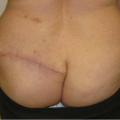
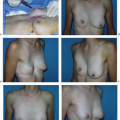
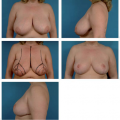
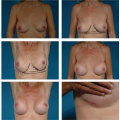
and using a significantly larger device, the surgeon should consider a standard gel implant or a round/elliptical device with a more form-stable filler when available. However, patients preferring this implant and having a relatively normal to tight skin envelope with less than 3 cm of skin stretch, patients who are extremely safety conscious, patients without ptosis, and younger patients are encouraged to consider the Style 410 device. In addition, patients with a constricted breast deformity and high fold are excellent candidates for a 410 with results that are difficult to match with standard devices, particularly in one stage (Fig. 116.7).
and using a significantly larger device, the surgeon should consider a standard gel implant or a round/elliptical device with a more form-stable filler when available. However, patients preferring this implant and having a relatively normal to tight skin envelope with less than 3 cm of skin stretch, patients who are extremely safety conscious, patients without ptosis, and younger patients are encouraged to consider the Style 410 device. In addition, patients with a constricted breast deformity and high fold are excellent candidates for a 410 with results that are difficult to match with standard devices, particularly in one stage (Fig. 116.7).
Tissue-Based Planning Principles
Careful assessments of a patient’s tissues including some basic measurements are important in determining the range of implants that will fit and match each patient’s breast and body. These tissue-based planning principles are important in all breast augmentation procedures, but they are absolutely critical when using shaped, form-stable devices. First, one evaluates the chest wall architecture, breast soft tissue volume, and skin stretch, noting the degree of asymmetry and whether any ptosis is present. These are important to document and show the patient. There are potentially more than 20 specific measurements of the breast; however, there are just a few that are vital in determining an implant range for each patient. This part of the process is both art and a science. We tend to be in an either-or society; however, the plastic surgeon must take into account all of these factors, including patient desires, in choosing a breast implant. I believe that the patient should decide the final implant size but that the plastic surgeon should set the range of what will fit each patient’s breast. There are consequences in going too large and creating malposition, symmastia, or other complications and also too small and not achieving an optimal fill or breast shape. The constant goal should be to continue to minimize surgical revisions for patients.
There are many current implant selection methods available; however, there are no current implant selector systems that take a patient and a plastic surgeon from an examination and evaluation through the selection of a specific range of devices that will fit each individual patient’s breast in a simple, straightforward, uncomplicated manner. Current systems are either extremely complicated or too overly subjective and not based on objective long-term deliverable data or results.
The key measurements and principles are as follows: determining base width of the breast, the breast tissue type, including stretch, and then using the sternal notch-to-nipple (SN-N)
measurement and nipple-to-inframammary fold (N-IMF) distance to help define the implant height. The breast base width is the keystone of the breast and the first critical measurement (Fig. 116.8). This measurement is best performed with calipers or a clear ruler, but is also very accurately measured with new three-dimensional camera systems. The breast base width determines the range of the breast implant width. In general, in the absence of a constricted or unusual situation, the breast implant should not exceed the breast width by more than 5 mm to less than 1 cm.
measurement and nipple-to-inframammary fold (N-IMF) distance to help define the implant height. The breast base width is the keystone of the breast and the first critical measurement (Fig. 116.8). This measurement is best performed with calipers or a clear ruler, but is also very accurately measured with new three-dimensional camera systems. The breast base width determines the range of the breast implant width. In general, in the absence of a constricted or unusual situation, the breast implant should not exceed the breast width by more than 5 mm to less than 1 cm.
Main principle: The base diameter of the device should generally not exceed the base diameter of the breast.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree