Perforator Flaps in Breast Reconstruction
Mark W. Clemens
Maurice Y. Nahabedian
Introduction
Current estimates from the American Cancer Society are that approximately 200,000 women in the United States will be diagnosed with breast cancer (1). While two thirds will receive lumpectomy and radiation, a significant number will undergo mastectomy. Of these, only 1 in 8 women will choose to undergo a reconstructive procedure, and of those, the vast majority will be reconstructed using prosthetic devices. This has been attributed to several factors that are related to both surgeon and patient issues. When prosthetic devices are used, it is usually because women will choose to have a simpler procedure with less recovery. Most plastic surgeons are comfortable with prosthetic reconstruction and are able to deliver excellent outcomes. When autologous tissues are used, it is usually because these women prefer to uses their own tissues and avoid the use of prosthetic devices. Most plastic surgeons are comfortable with pedicle flaps, and only a relative few are comfortable with the microvascular techniques. The use of microvascular perforator flaps for breast reconstruction has been receiving considerable interest and attention. At the present time, only a handful of plastic surgeons offer this procedure, although patient demand and surgical capabilities are increasing.
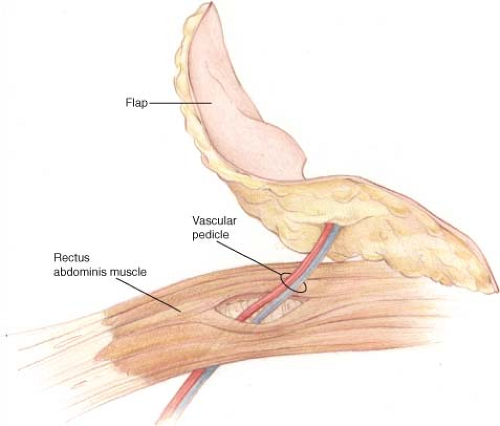
Breast reconstruction using autologous tissue has traditionally been performed using musculocutaneous flaps. Although musculocutaneous flaps can create a beautiful breast mound, there is some additional morbidity associated with harvesting the donor-site muscle (2,3). The concept of perforator flaps evolved as a means of reducing donor-site morbidity by only harvesting the adipocutaneous portion of the flap and leaving the muscle in its natural position (Fig. 61.1). Breast reconstruction with perforator flaps has expanded our reconstructive options for women following mastectomy.
The surgeon credited with performing the first perforator flap is Koshima in 1989 (4). Over the years, a number of surgeons and breast centers have offered perforator flaps to their patients, demonstrating that the technique can result in a soft and “natural-appearing” breast. Studies have demonstrated several benefits of perforator flaps, including reliable transfer of skin and fat, no muscle loss with minimal functional impairment, decreased postoperative pain, and shortened hospital stays (5). This success is due in part to our understanding of angiosomes and the anatomy of main perforating branches. Further advances in perforator flap surgery are dependent upon the knowledge of vessel variations and the reliability of vascular territories. While many perforator flaps have been described, this chapter details the flaps of the lower abdomen, buttock, back, and lateral thigh and provides an overview of the various perforator flaps available for breast reconstruction. Currently, the common flaps include the deep inferior epigastric perforator (DIEP) flap, superficial inferior epigastric artery (SIEA) flap, superior gluteal artery perforator (SGAP) flap, inferior gluteal artery perforator (IGAP) flap, anterior lateral thigh (ALT) flap, thoracodorsal artery perforator (TDAP) flap, and intercostal artery perforator (ICAP) flap. This introductory chapter highlights some of the unique features and salient aspects of these flaps. A detailed review of the various flaps and harvesting techniques is provided in subsequent chapters.
Indications
When a patient is deemed a suitable candidate for autologous reconstruction, a perforator flap can be considered in most situations. Current indications include immediate and delayed reconstruction of the postmastectomy deformity, the partial mastectomy deformity, the failed prosthetic reconstruction, and congenital breast deformities. The decision to proceed with perforator flap reconstruction may be patient driven. A patient should be adequately informed of the risks associated with perforator flaps, such as total failure, partial failure, and fat necrosis. The particular perforator flap selected is based on the size and volume of the natural breast as well as the amount of tissue available at the donor site. This is usually achieved by visual assessment; however, some have argued that a more accurate assessment can be calculated using three-dimensional imaging techniques (6). When selecting a particular perforator flap, a surgeon should be mindful of appropriate tissue match, length of the vascular pedicle, caliber of recipient vessels, and surface area, volume, and thickness of the flap. Flaps with a short vascular pedicle may require a vein graft; however, with internal mammary recipient vessels, this is generally not a problem. In our practice, women with a breast volume less than 1,000 cc are potential candidates for DIEP or free transverse rectus abdominis myocutaneous (TRAM) flap reconstruction, whereas women with a breast volume greater than 1,000 cc are better candidates for the free TRAM flap (7). Large-volume (>1,000 cc) breast reconstruction may be associated with an increased risk of complications related to inadequate vascular perfusion. In these cases, the inclusion of a muscle segment with several perforators (large and small) may potentially offer a perfusion advantage.
Preoperative imaging to determine the location and size of the abdominal perforators has been shown to be useful. This can be performed using duplex ultrasound or computed tomography angiography (8). Imaging may aid in determination of perforator location and surgical planning. It can be used for abdominal flap, gluteal flaps, and all flaps in which a source vessel can be cannulated by an interventional radiologist. It is useful in cases in which prior abdominal operations may obscure the location or patency of a perforator. When perforator caliber or location is unfavorable based on the preoperative imaging, a perforator flap should not be performed. In some cases when the abdomen is imaged, the SIEA may be strongly visualized and preferred over the deep system.
Contraindications
Contraindications to perforator flap reconstruction are similar to those of other types of free tissue transfer and include previous incisions across donor sites that might compromise vascularity and the amount of available tissue. In the abdomen, a previous Pfannenstiel incision usually does not compromise the integrity of the deep vessels but will divide the superficial system. Preoperative imaging may be useful in these patients.
Patient comorbidities are important to assess because flap and patient survival may be dependent on the clinical status of the patient. Advanced age, compromised nutritional status, tobacco use, and presence of underlying comorbidities (e.g., diabetes mellitus, cardiopulmonary disease, and peripheral vascular disease) are not absolute contraindications but may contribute to partial flap loss, fat necrosis, and delayed wound healing (9). Women with a strong history of tobacco use may be better candidates for a musculocutaneous flap to include more perforators and minimize the morbidity related to vasoconstriction. Morbidly obese women witha large abdominal pannus are usually discouraged from proceeding with abdominal flap reconstruction (10). Paradoxically, patients status post massive weight loss may be particularly suitable candidates for cutaneous perforator-based reconstruction because vessel caliber remains irreversibly enlarged despite weight reduction (11,12). Patients with poorly controlled diabetes mellitus require adequate glucose control prior to free tissue transfer. Surgical clearance by a medical physician is recommended for patients with multiple medical problems.
Perforator Flaps
Deep Inferior Epigastric Perforator Flap
The DIEP flap was introduced in 1989 and popularized in 1994. The benefit of this flap when compared to the pedicle or the free TRAM is that the rectus abdominis muscle is not removed. The DIEP flap can produce a soft and natural-appearing breast that is usually associated with an improvement in abdominal contour and a minimal change in abdominal strength (3,4,13). For patients who present with a sufficient quantity of skin and fat overlying the abdomen, the DIEP flap is ideal for breast reconstruction. Patients requiring simultaneous bilateral reconstruction are particularly suited for DIEP flap reconstruction without sacrifice of the rectus muscle.
The vascularity of the DIEP flap is derived from the deep inferior epigastric artery and vein, which are branches from the external iliac artery and vein. The deep system has multiple perforators that supply the anterior abdominal wall. The DIEP flap is usually designed to incorporate one to three of these perforators. The DIEA courses along the posterior surface of the rectus abdominis and enters either the lower (17%), middle (78%), or upper (5%) one third of the muscle (14). The flap is raised by following these perforators to a single pedicle with preservation of the rectus muscle and rectus sheath. Two venae comitantes usually accompany the artery.
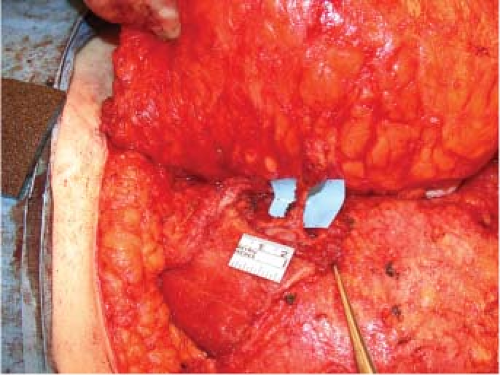
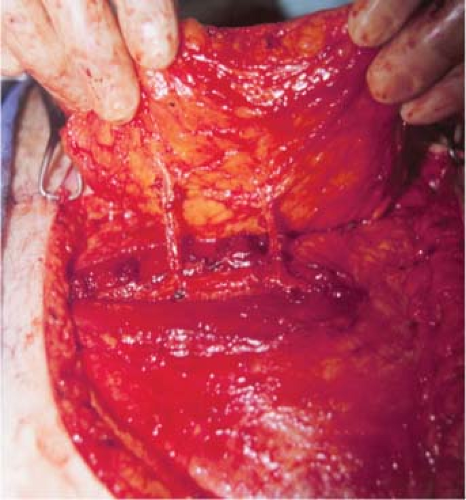
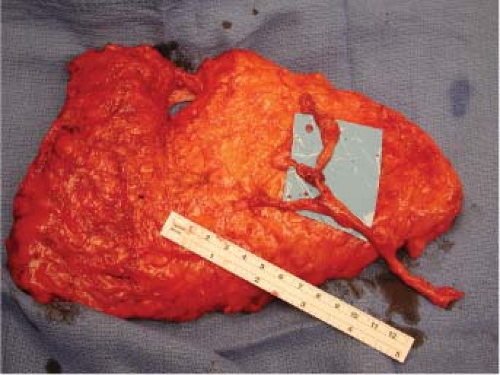
The vascular anatomy and architecture of the anterior abdominal wall will often dictate the safest and most efficacious lower abdominal flap to use. The DIEP flap can be raised on a single or multiple perforators. Nahabedian et al. reviewed 88 DIEP flaps and examined the number of perforators included
with the flaps. They demonstrated that one perforator was included in 76%, two perforators were included in 22%, and three perforators were included in 2% (Figs. 61.2 to 61.4) (15). The deep inferior epigastric artery may have a dominant medial (18%), central (28%), or lateral (54%) branch pattern. In approximately 5% of cases the perforator may course around the medial border of the rectus abdominis muscle (Fig. 61.5). Lateral perforators tend to course more perpendicularly, which may ease dissection. The majority of perforators are located within 8 cm of the umbilicus, 2 cm above and 6 cm below the umbilicus. Doppler imaging has determined the average mean diameter of the deep inferior epigastric artery to be 3.6 mm (range, 2.8 to 5 mm) (16,17). Average pedicle length is 10.3 cm but may be quite variable. Several segmental nerves enter the rectus abdominis at the junction of the lateral and central segments. Small, type 1 nerves demonstrate overlapping innervation from adjacent nerves and may be sacrificed without functional detriment. Single large type 2 nerves at the level of the arcuate line innervate the entire width of the rectus muscle and may contribute to donor-site morbidity and abdominal bulge if sacrificed (18). Figures 61.6 and 61.7 illustrate a completed unilateral DIEP flap reconstruction.
with the flaps. They demonstrated that one perforator was included in 76%, two perforators were included in 22%, and three perforators were included in 2% (Figs. 61.2 to 61.4) (15). The deep inferior epigastric artery may have a dominant medial (18%), central (28%), or lateral (54%) branch pattern. In approximately 5% of cases the perforator may course around the medial border of the rectus abdominis muscle (Fig. 61.5). Lateral perforators tend to course more perpendicularly, which may ease dissection. The majority of perforators are located within 8 cm of the umbilicus, 2 cm above and 6 cm below the umbilicus. Doppler imaging has determined the average mean diameter of the deep inferior epigastric artery to be 3.6 mm (range, 2.8 to 5 mm) (16,17). Average pedicle length is 10.3 cm but may be quite variable. Several segmental nerves enter the rectus abdominis at the junction of the lateral and central segments. Small, type 1 nerves demonstrate overlapping innervation from adjacent nerves and may be sacrificed without functional detriment. Single large type 2 nerves at the level of the arcuate line innervate the entire width of the rectus muscle and may contribute to donor-site morbidity and abdominal bulge if sacrificed (18). Figures 61.6 and 61.7 illustrate a completed unilateral DIEP flap reconstruction.
Superficial Inferior Epigastric Artery Flap
The SIEA flap is a further refinement in an attempt to limit the morbidity of the abdominal wall donor site. Some controversy exists over whether a DIEP flap is superior to a TRAM flap for
avoiding donor-site morbidity (19). Advances in muscle-sparing techniques now leave much of the rectus muscle intact and viable during the harvest of a free TRAM. In addition, abdominal bulge, hernia, and partial loss of rectus function following DIEP flap harvest can be as high as 10% to 20% and has been attributed to nerve sacrifice during perforator dissection (20). The SIEA flap obviates this potential problem by maintaining the full extent of pedicle dissection just deep to the dermis and following a course inferomedially into the deeper fatty tissue of the groin without violation of rectus fascia (Fig. 61.8).
avoiding donor-site morbidity (19). Advances in muscle-sparing techniques now leave much of the rectus muscle intact and viable during the harvest of a free TRAM. In addition, abdominal bulge, hernia, and partial loss of rectus function following DIEP flap harvest can be as high as 10% to 20% and has been attributed to nerve sacrifice during perforator dissection (20). The SIEA flap obviates this potential problem by maintaining the full extent of pedicle dissection just deep to the dermis and following a course inferomedially into the deeper fatty tissue of the groin without violation of rectus fascia (Fig. 61.8).
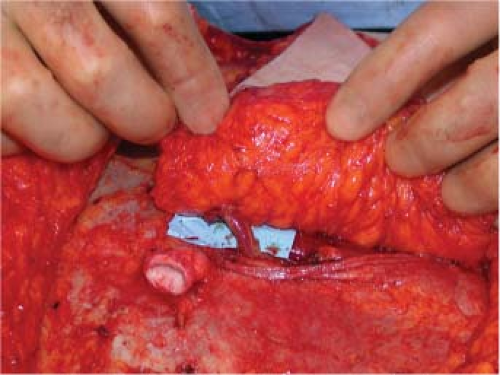 Figure 61.5. In approximately 5% of cases the perforating vessel will course around the medial border of the rectus abdominis muscle as depicted. In these case no myotomy is necessary. |
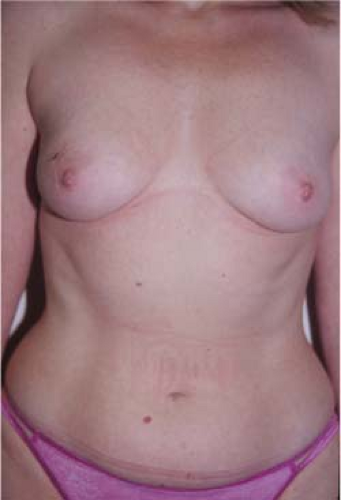 Figure 61.6. Preoperative image of a patient with right breast cancer.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|