Reconstruction of the Irradiated Breast
Scott L. Spear
Matthew L. Iorio
Pranay M. Parikh
Introduction
Radiation therapy as an adjunct to tumor resection has optimized oncologic outcomes for patients with breast cancer and has had a significant impact on patients’ reconstructive options and outcomes. For the reconstructive surgeon, radiation therapy has increasingly become a consideration in patient care, and the surgeon must be prepared for a variety of scenarios in which irradiation can occur prior to reconstruction, during reconstruction, or years after reconstruction. The reconstructive plan must take into account the immediate and late effects of radiation, as irradiated tissue differs from unirradiated tissue in its pliability, vascularity, and healing capacity, all of which can be crucial to achieving a satisfactory and lasting reconstruction.
Oncologic Need for Radiotherapy
The use of radiation therapy as a part of breast cancer treatment dramatically increased following two landmark studies demonstrating the clinical utility in reducing the risk of locoregional cancer recurrence and improving survival in high-risk premenopausal women.
The first study, the multicenter Danish Breast Cancer Cooperative Group 82b trial, included 1,708 women who had undergone mastectomy for stage II or III breast cancer (1). Patients were randomized to receive chemotherapy plus irradiation of the chest wall and regional lymph nodes, or chemotherapy alone. Both groups were longitudinally evaluated for two types of cancer recurrence: locoregional recurrence, and distant metastasis without locoregional recurrence. At a median follow-up of 9.5 years, locoregional cancer recurrence was seen in 32% of patients receiving chemotherapy alone and in 9% of patients receiving radiation and chemotherapy. The benefit of radiation was noted as a decrease in locoregional recurrence as well as an increase in disease-free and overall survival. The disease-free probability of survival at 10 years was increased to 48% in women who received radiation plus chemotherapy, as compared to 34% in women who received chemotherapy alone. This survival benefit was attributed to better control of locoregional disease, with a subsequent decrease in the probability of distant metastasis, and ultimately in breast cancer mortality (2).
In a similar study from Vancouver, British Columbia, Canada, 318 premenopausal women with stage I or II breast cancer were randomized to receive radiation and chemotherapy or chemotherapy alone and were followed for breast cancer recurrence (3). Recurrence was observed in 42% of the women treated with radiation and chemotherapy and in 63% of the women treated with chemotherapy alone. In addition, women treated with chemotherapy and radiotherapy were found to have a 29% reduction in the rate of mortality from breast cancer.
Despite these demonstrated recurrence and survival benefits, the potential morbidity of radiation remains high, and the indications and patient-specific criteria for the use of radiation therapy are still being studied. The American Society of Clinical Oncology recommends postmastectomy radiation therapy for patients with four or more positive lymph nodes to achieve better locoregional control and possibly improve survival (4). Radiation is also recommended for patients with advanced primary cancers (stage T3 or greater), as these patients remain at risk for locoregional recurrence after mastectomy despite the use of adjuvant chemotherapy and hormone therapy.
Local Tissue Effects of Radiotherapy
The mechanism of action of radiation therapy is the disruption of cellular architecture and replication, with resultant cell death. Unfortunately, these effects are not specific to cancer cells and can create extensive collateral damage to surrounding normal tissue present in the treatment field, including critical structures such as the heart and lungs.
Tissue damage occurs from a biphasic process, with an acute and a chronic phase. Acute effects occur within days to weeks and affect the cycle of cellular turnover and result in cellular death. These effects manifest as edema, followed by capillary leak and inflammation. The late effects are a product of this inflammatory response and include local tissue fibrosis as a result of the replacement of normal adipose tissue with collagen (5).
Based on our experience, we believe that if the breast undergoes irradiation, one can reasonably expect changes as a result of the radiation exposure. These effects can include pigmentation changes, atrophy of subcutaneous fat, contracture and fibrosis of skin and muscle, and delayed wound healing. Such consequences are not limited to the local tissues, however, as autologous tissue transfers are affected by similar types of fibrosis and contracture when irradiated. Even prosthetic materials are indirectly affected, with resultant increased capsular contracture around irradiated prosthetic implants.
In a study of partial breast irradiation by Chen et al., 199 consecutive patients were given postmastectomy radiation therapy using interstitial brachytherapy (6). At the time of therapy all patients had stage I to II breast carcinoma. The mean follow-up was 6.4 years. The study identified fibrosis and hypopigmentation as the only two sequelae that stabilized at the 2-year mark. Fat necrosis continued to increase through the 5-year mark, with 1% at 6 months and 11% at five years. Breast edema decreased from 50% at 6 months to 6% by 5 years, and similar reductions in erythema and breast pain were seen. The study demonstrated that radiation sequelae are not limited to the perioperative period and can continue and possibly delay reconstructive success for a number of years.
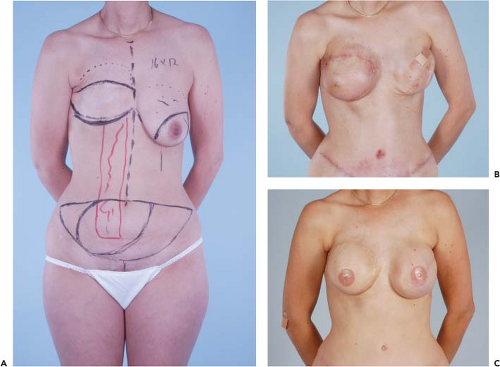
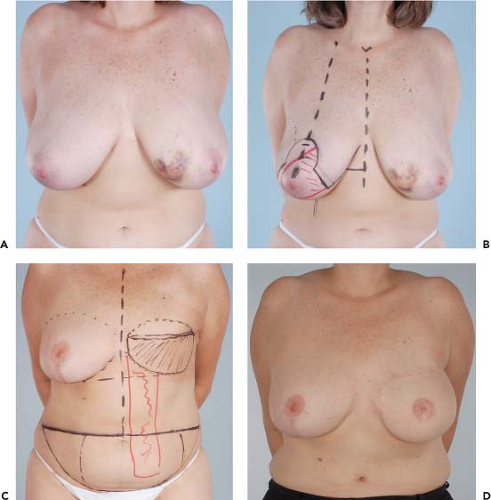
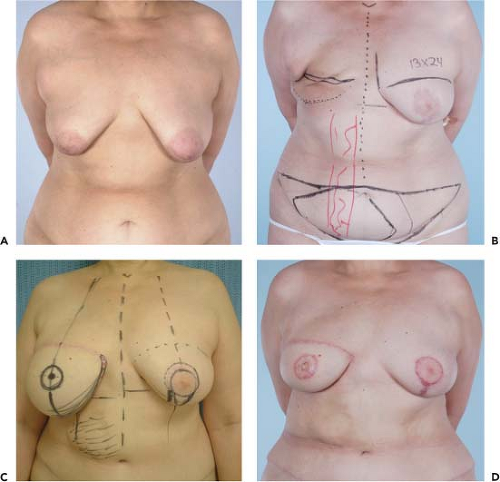
Figures 49.1 to 49.3 illustrate the effects of radiation therapy in conjunction with transverse rectus abdominis myocutaneous (TRAM) flap reconstruction. As the cases illustrate, the effects of radiation therapy are often extensive. In addition, the long-term effects of radiation dermatitis, fat necrosis, and capsular contracture demand a reconstructive technique that is long lasting and aesthetically resilient (7).
Timing of Radiotherapy
In the context of breast cancer treatment and reconstruction, radiation therapy is administered typically in one of three conditions: (a) as part of breast-conserving therapy, (b) postmastectomy with delayed or immediate reconstruction, and (c) prior to mastectomy and reconstruction. Radiation is used for adjuvant purposes or for the treatment of locoregional recurrent disease (8). However, the reconstruction may alter how the irradiation can be delivered (9,10), and the radiation may compromise the reconstruction and lead to a larger number of revision procedures (11).
Controversy exists, then, as to the safest, most reliable, and most aesthetically viable option for reconstruction following radiation. Chang et al. reviewed 1,000 consecutive cases of implant-based reconstructions to define the role of combined autologous tissue and implant-based reconstruction (12). In the study group, 704 patients were identified who had prosthetic reconstruction and did not receive preoperative or postoperative radiation. The rate of reconstructive failure in this group was 10.9% (n = 77) as compared to failure rates of 42.4% and 56.4% in patients receiving preradiation or postradiation therapy, respectively.
In a study of 888 patients from Emory University, Losken et al. examined how radiation affects the number of revisions a patient typically undergoes after initial reconstruction (Table 49.1) (11). Of the 888 patients who underwent breast reconstruction, 107 underwent radiation therapy. In their study, patients receiving radiation after reconstruction required more
revisions in both unilateral (4.6 vs. 3.9, p < 0.001) and bilateral reconstruction (6.4 vs. 5.7, p < 0.032).
revisions in both unilateral (4.6 vs. 3.9, p < 0.001) and bilateral reconstruction (6.4 vs. 5.7, p < 0.032).
Table 49.1 Number of Secondary Procedures Stratified by Radiation Therapy | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
As a response to the elevated revision rate in the radiated reconstruction, Kronowitz et al. fashioned the two-stage approach to autologous reconstruction, or “delayed-immediate reconstruction” (13). In the first stage, a skin-sparing mastectomy is performed with a subpectoral insertion of a textured saline tissue expander. If the patient does not require radiation therapy after definite pathologic staging, the second stage of definitive reconstruction is started. In contrast, patients who require postmastectomy radiation undergo treatment and have a delayed reconstruction. By the use of this approach, patients who do not require postmastectomy radiation can achieve aesthetic outcomes similar to those with immediate reconstruction, and patients who require postmastectomy radiation therapy can avoid the aesthetic and radiation delivery problems that can occur after an immediate breast reconstruction (13).
In effect, the expander holds the tissue envelope while final pathology is reviewed and during possible irradiation so that contracture of tissue following treatment may be minimized.
In effect, the expander holds the tissue envelope while final pathology is reviewed and during possible irradiation so that contracture of tissue following treatment may be minimized.
In the study of Kronowitz et al., patients who were treated with adjuvant chemotherapy prior to radiation therapy received chemotherapy with the expander fully inflated. The expander was then deflated before initiation of radiation therapy, thereby restoring the preinflation contour. This reduction in profile addresses the radiation oncologists’ concern for minimizing the radiation injury to the heart and lungs while maximizing the radiation dose delivered to internal mammary nodes. The expander was then serially expanded once the acute radiation-induced skin desquamation resolved.
Prosthetic-Based Reconstruction
Spear and Onyewu performed a retrospective review of the effect of radiation therapy on staged reconstruction using tissue expanders followed by saline-filled implants (14). The study was performed using 40 consecutive patients who had undergone two-stage expander and saline implant breast reconstruction and radiation over a 7-year period. A randomly selected group of 40 other two-stage, saline-filled implant reconstructions from the same surgeon and time period served as controls. The radiated group was further divided into four subgroups: (a) breast conservation therapy followed by staged reconstruction (n = 7), (b) mastectomy and radiation followed by staged reconstruction (n = 9), (c) mastectomy with delayed reconstruction after radiation therapy (n = 19), and (d) radiation after completion of the staged reconstruction (n = 5).
The primary outcomes followed were the number of flap procedures and types of complications in each group (Table 49.2). In the irradiated group, 47.5% (19 of 40) of the staged reconstruction needed the addition or replacement by a flap procedure for salvage or improved aesthetics (14). However, in the nonirradiated group, only 10% (4 of 40) required a flap procedure.
Table 49.2 Two-Stage Implant Reconstruction With and Without Radiation | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
Four types of complications (capsular contracture, extrusion, deflation, and infections) were studied (Table 49.2). The incidence of complications in the irradiated group was 52.5% (21 of 40), which was significantly higher than within the control group at 10% (4 of 40). In terms of aesthetics, the irradiated implant reconstructions were judged to be poorer than the controls. The study concluded that breast reconstruction with implants that undergo radiation are often aesthetically inferior and are more likely to require salvage or autologous tissue support (14). As such, it is usually preferred when reconstructing the irradiated breast to use a combination of autologous tissue and possibly a prosthetic device, as a prosthetic device without adequate soft tissue coverage and protection may suffer the consequences of increased contracture and poor aesthetic outcome. Figures 49.4 and 49.5 demonstrate implant-based reconstructions, which can have severe and marked radiation-induced skin changes and expander contracture that need revision at the time of definitive implant placement.
Autologous Tissue Reconstruction: Pedicled Transverse Rectus Abdominis Myocutaneous Flap
In an effort to determine the impact of radiation on pedicled TRAM reconstructions, several studies have evaluated outcomes and complication rates following breast irradiation. Spear et al. reviewed 171 pedicled TRAM reconstructions in 150 patients during an 11-year period (15). The three groups consisted of the control group of TRAM patients without radiation therapy (n = 91), those who received radiation prior to TRAM reconstruction (n = 42), and those who received radiation post TRAM reconstruction (n = 38). The complications that were examined included infection, delayed wound healing, fat necrosis, flap loss, hematoma, and seroma formation (Table 49.3). There were no statistically significant differences in the incidence of these complications among the three groups (15). However, there was an increased incidence of fat necrosis in the group that received radiation prior to pedicled TRAM reconstruction. Cosmesis was also judged, and it was determined that compared with controls, those with radiation therapy had poorer aesthetic outcomes and symmetry, increased pigmentation, and increased contracture (Table 49.4).
Other studies on the effect of radiation after TRAM flap reconstruction show similar results. Williams et al. evaluated 19 patients who received radiation therapy for locoregional control of cancer after they underwent reconstruction with a
TRAM flap (16). This group was compared with a group of 108 patients who received radiation prior to TRAM reconstruction. The control group in this study was a group of 572 patients who underwent TRAM reconstruction alone without radiation therapy. Overall, Williams et al. found that there was a higher complication rate in those who had their TRAM flaps radiated than in those who received preoperative radiation therapy (Table 49.5). They also reported that the incidence of fat necrosis was greater in both groups that received radiation than in the control group.
TRAM flap (16). This group was compared with a group of 108 patients who received radiation prior to TRAM reconstruction. The control group in this study was a group of 572 patients who underwent TRAM reconstruction alone without radiation therapy. Overall, Williams et al. found that there was a higher complication rate in those who had their TRAM flaps radiated than in those who received preoperative radiation therapy (Table 49.5). They also reported that the incidence of fat necrosis was greater in both groups that received radiation than in the control group.
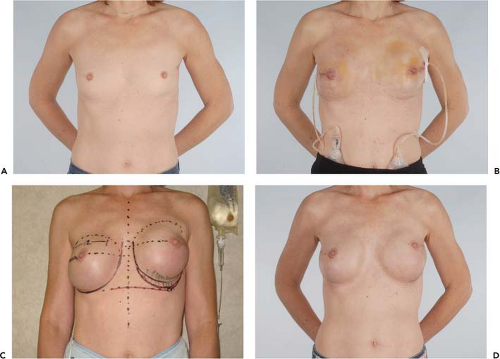 Figure 49.4. A 45-year-old woman with left breast cancer treated with lumpectomy and irradiation. She subsequently had bilateral nipple sparing mastectomies with immediate reconstruction using tissue expanders and an acellular dermal matrix. Final reconstruction was achieved following implant exchange. A: Preoperative photo. B: Result following nipple-sparing mastectomies and bilateral expander placement. C: Preoperative planning for implant exchange and correction of prominent inferior-pole contracture on the irradiated left side. D: Final result following implant exchange.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|