Introduction
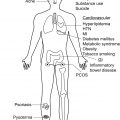
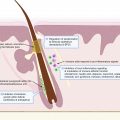
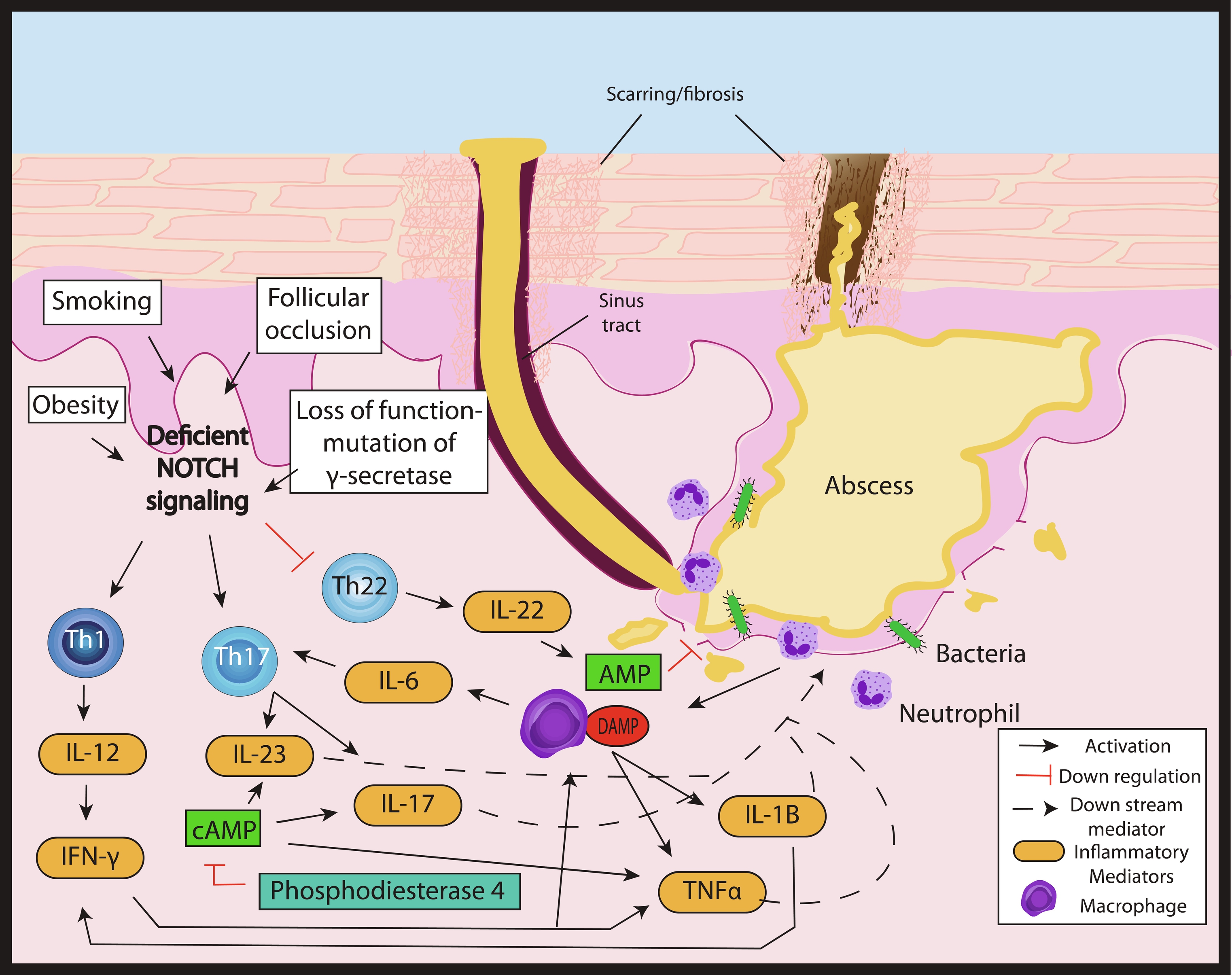
Hidradenitis suppurativa (HS) is a chronic inflammatory disorder with a complex, multifactorial, and incompletely elucidated pathophysiology. Among relevant host factors are a number of important inflammatory pathways, including increased production of oxygen free radicals, enhanced expression of toll-like receptors and release of pro-inflammatory cytokines, increased tumor necrosis factor (TNF)-α expression, activation of the interleukin-23 (IL-23)/T helper-17 (TH-17) pathway, overproduction of interleukin-1 (IL-1), and others ( Fig. 18.1 ). Historically, antibiotic and surgical treatments were the mainstay of HS therapy, but in the last decade a growing number of biologic therapies which modulate these inflammatory pathways have become available and have been investigated for treatment of moderate-to-severe HS. The appropriate candidate for biologic therapy has moderate or severe HS (correlating with Hurley stage II or III) and has either failed appropriate non-biologic therapy (e.g., 3 months or longer of doxycycline) or has inflammatory HS, which would benefit from the use of a biologic as initial therapy. This chapter will review in detail the evidence for these targeted therapeutics, including adverse effects, monitoring recommendations, treatment pearls, and considerations for successful treatment of HS in the setting of relevant patient comorbidities ( Table 18.1 ).
| Biologics and Small Molecules | Mechanism of Action | Comorbidity Use (FDA-approved for use in below conditions) | Contraindication | Efficacy Data and Level of Evidence | Major Adverse Events | Additional Notes |
|---|---|---|---|---|---|---|
| Adalimumab | Fully humanized IgG1 monoclonal antibody against TNF-α | FDA approved for use in: Rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), Crohn’s disease and ulcerative colitis (IBD), psoriasis (PsO), hidradenitis suppurativa (HS) uveitis | Demyelinating conditions such as multiple sclerosis (MS); congestive heart failure (CHF); Lupus (SLE) | A, I |
|
|
| Infliximab | IgG1 monoclonal antibody against TNF-α | IBD, RA, AS, PsO, PsA | MS; Relative contraindication: CHF and SLE | B, II |
|
|
| Anakinra | IgG1 monoclonal antibody IL-1-α and 1β inhibitor | RA, Cryopyrin-Associated Periodic Syndromes (CAPS) | Known hypersensitivity to E. coli -derived proteins, Anakinra | B, II |
|
|
| Canakinumab | IgG1 monoclonal antibody to IL-1β inhibitor | CAPS, Muckle-Wells (MW), Familial Cold Autoinflammatory Syndrome (FCAS) | Known hypersensitivity to Canakinumab | C, III |
|
|
| Ustekinumab/IL12/23 inhibitors | IgG1 monoclonal antibody binds to p-40 subunit of both IL-12 and IL-23 | PsO, PsA, IBD | Known hypersensitivity to Ustekinumab | B, II |
|
|
| Guselkumab/p19 inhibitors | IgG1 monoclonal antibody that binds the P19 subunit of IL-23 | PsO | None | C, III |
|
|
| Secukinumab, Ixekizumab/IL17 inhibitors | IgG1 monoclonal antibody that binds IL-17A | PsA | Known hypersensitivity to Secukinumab, Ixekizumab; IBD | C, III |
|
|
| Tofacitinib and janus kinase (JAK) inhibitors | JAK 3 inhibitor | RA, PsA, UC | Patients with severe hepatic impairment, known hypersensitivity | C, III |
|
|
Tumor Necrosis Factor Inhibition
Adalimumab (TNF-α Inhibitor)
Adalimumab is the first FDA-approved medication for the treatment of HS and is thus the first-line agent for treatment of moderate or severe HS which has failed to respond to non-biologic therapies. Efficacy was established with publication of the Pioneer I and II trials in 2016. Pioneer I and II demonstrated a modest but significant improvement in disease for roughly half of patients. The two trials included anti-TNF treatment-naive patients with Hurley Stage II or III disease and at least 3 inflammatory nodules. Treatment included a loading dose followed by maintenance dosing at 40 mg weekly. HS Clinical Response, termed the HiSCR, was defined as a 50% reduction in inflammatory nodule count. HiSCR was achieved in 41.8% of patients in the adalimumab arm of Pioneer I and 58.9% of patients in the adalimumab arm of Pioneer II, compared to 26% and 27.6% of patients in the placebo arms, respectively. Health-related quality-of-life measures also improved significantly among patients in the treatment arm, including better pain numeric rating scale scores and improvements in Dermatology Life Quality Index (DLQI) measures. Based on this and subsequent data, adalimumab has become the mainstay of therapy for patients with moderate-to-severe HS following antibiotic failure.
Pioneer I and II demonstrated efficacy in HS through the use of higher dosages than were previously standard for other inflammatory conditions; psoriasis, rheumatoid arthritis, inflammatory bowel disease (IBD), and psoriatic arthritis all utilize every-other-week maintenance dosing of adalimumab. However, in studies comparing weekly with every-other-week dosing of adalimumab for HS, patients receiving every-other-week dosing (or decreasing to every-other-week dosing after initial response to weekly dosing) experienced reduced efficacy. Standard treatment based on these and other trials has established the need for a loading dose of 160 mg at week 0, followed by 80 mg at week 2, and then continued use of 40 mg weekly thereafter beginning at day 28.
While adalimumab has clear efficacy for the treatment of HS, outcomes from the PIONEER trials were also notable for the number of non-responders. Roughly half of PIONEER I and PIONEER II patients did not achieve HiSCR (50% disease response) with the use of weekly adalimumab. Patients experiencing a partial response to therapy at week 12 frequently achieve HiSCR by week 36 with continued therapy, but those who do not show at least a partial response by week 12 are unlikely to benefit from continued treatment and should be considered for alternative therapies. Among responders, continued weekly dosing of adalimumab is considered the most effective strategy, yet those who achieve HiSCR generally continue to have flares and waxing and waning disease activity.
Infliximab (TNF-α Inhibitor)
Patients with inadequate response to therapy with adalimumab frequently benefit from a switch to infliximab. While infliximab has not been studied in a randomized controlled trial for HS comparable in size to those for adalimumab, it nevertheless offers an evidence-based alternative for treatment of moderate or severe HS, as outlined in the European and American treatment guidelines and a Cochrane Review. Infliximab is an anti-TNF-α therapy administered intravenously in a weight-based fashion. Loading doses at weeks 0, 2, and 6 are followed by maintenance dosing every 4 to 8 weeks thereafter. Randomized controlled trials of Infliximab have demonstrated significant disease improvement over placebo; however, sample sizes are small.
Weight-based dosing is a potential advantage of infliximab, as is the ability to titrate the dose to effect. In general, higher doses of the medication may be more effective for more severe, inflammatory HS. Published data support the need for dose escalation in most patients (64%; 34/52), with treatment response and achievement of stable dosing generally occurring at 10 mg/kg every 6 to 8 weeks. Other data suggest most (71%; 17/24) patients receiving 7.5 mg/kg every 4 weeks (after loading) achieve a good (HS PGA 0 to 2) clinical response, while dose escalation to 10 mg/kg every 4 weeks is often (50%; 6/12) successful in those who do not.
Other Anti-Tumor Necrosis Factor Therapies
Etanercept, 50 mg injected subcutaneously weekly or twice weekly, was studied in two small prospective studies versus placebo and failed to yield significant disease improvement. Based on available evidence, it is not recommended for treatment of HS.
Evidence for the use of golimumab in HS is limited to two case reports, one positive and one negative. Use of certolizumab is anecdotal. Despite a lack of data, these agents may be useful in some cases, particularly in the setting of a comorbid condition (e.g., rheumatoid arthritis, IBD) or a prior intolerance of alternative therapies.
Adverse Reactions to Anti-Tumor Necrosis Factor Therapies
Injection Site Reactions
Injection site reactions are a common complication of adalimumab use, characterized by erythematous patches and plaques, generally round and edematous, arising minutes to hours following drug administration. Reactions generally occur within the first month of treatment and last 3 to 5 days after each exposure. They are thought to constitute a type I hypersensitivity reaction and should be treated as such. Reactions can be treated with topical steroids, antihistamines, nonsteroidal antiinflammatory drugs (NSAIDs) or acetaminophen, and ice. Some severe reactions cause significant pain and edema and may require medication discontinuation. In these cases, a switch to a citrate-free formula of adalimumab can help control injection site reactions while sparing the patient the need to switch to an alternative therapy.
Infusion Reactions
Infusion reactions (IR) to infliximab are well-documented given the drug’s extensive use in other rheumatologic, dermatologic, and gastrointestinal conditions. IRs can be divided into early reactions, which occur peri-infusion, and late reactions, which occur 24 hours following infusion. Immediate IRs include symptoms such as pruritus, dyspnea, flushing, and headache, as well as anaphylaxis. These reactions are thought to be mediated via host activation of complement, IgE, and mast-cell degranulation in the setting of the TNF immune globulin. Mild infusion reactions such as transient flushing, myalgia, and pruritus are managed with attenuation of infusion rate, while moderate reactions such as fever, urticaria, and hypertension are treated with infusion interruption, oral antihistamine and acetaminophen therapy, and resumption of infusion at slower rates. Severe IRs, including bronchospasm and hypotension, are managed promptly with typical anaphylaxis response and discontinuation of the infusion. Patients can be re-challenged with test doses and graded drug infusion if infliximab is deemed warranted. Because immediate IRs are common, infliximab is generally ordered with as needed medication available and may be given following pre-medication with corticosteroids, antihistamines, and anti-pyretics. Montelukast may help prevent some respiratory symptoms. In the case of severe IRs with intended re-challenge, input from allergy/immunology may help guide premedication protocols. While these premedication protocols are routinely employed in the setting of infliximab, evidence for their use is limited.
Strategies for Addressing Human Anti-Chimeric Antibody Formation
Unlike immediate IRs, late IRs tend to develop as a result of immune complex deposition and develop in the setting of high human anti-chimeric antibodies (HACAs), prolonged drug-free intervals, and episodic treatment. Because they impede the efficacy of infliximab (or adalimumab), preventing HACA development is critical. Therefore, missed doses and prolonged, drug-free intervals should be avoided to prevent development of HACAs. Low-dose methotrexate (MTX) (5 to 10 mg weekly), as well as azathioprine (50 mg daily), have been used in both the dermatologic and gastroenterologic contexts to prevent the development of HACAs. Initiation of low-dose methotrexate after development of anti-chimeric antibodies is an alternative strategy which may successfully suppress HACAs and enable continued use of infliximab. HACA formation should be suspected when a previously successful regimen ceases to be effective. In such instances, testing for antibody level and drug titer may be advised to guide next steps in management.
Infection and Cardiovascular Risk
The risk of serious infection is greater in patients treated with anti-TNF therapy as compared to those with the same condition treated with other, non-biologic therapies. This includes bacterial infections (particularly pneumonia), tuberculosis and other mycobacterial infections, invasive fungal infections, as well as viral infections (namely, hepatitis B reactivation). Screening for latent tuberculosis and hepatitis B infection should occur before initiation of TNF-α inhibitor therapy; tuberculosis screening is generally repeated annually.
Some data resulting from randomized trials investigating TNF-α inhibitors as possible treatment for heart failure (HF) suggest they may be associated with HF exacerbation. Based on available evidence, patients with mild (New York Heart Association [NYHA] class I/II), stable HF may safely receive anti-TNF therapy. However, their use should be avoided in those with uncontrolled, symptomatic NYHA class III/IV disease.
Other Biologic Therapies
The efficacy of anti-TNF therapy in patients with HS, along with elevated serum TNF levels, have highlighted the role of this pathway in the pathophysiology of HS. However a significant number of patients with HS do not respond to anti-TNF therapy, suggesting the disease cannot be fully explained or controlled via a single inflammatory pathway alone. Research to date has demonstrated that Th17-associated cytokines are increased in HS, including IL-1, IL-17, and IL23. In kind, CD161, a marker of Th17 lineage T-cells, is significantly elevated in lesional skin (LS) and peri-lesional skin (PLS) in patients with HS, as compared to healthy controls. T-regulatory (Treg) cells are also elevated in HS, LS, and PLS; however, the ratio of Th17 to Treg cells is highly skewed in HS tissue toward Th17 cells, suggesting immune dysregulation plays a significant role in HS inflammation. IL-1β and TNF have themselves demonstrated differential concentrations in draining lesions, depending on the patient.
IL-1 inhibitors, IL-17 inhibitors, and IL-23 inhibitors are generally the next rungs on the therapeutic ladder when patients fail anti-TNF therapy. Oddly, many of these treatments can also paradoxically induce hidradenitis in certain patients and worsen the course for others. The following section aims to review these therapies, their side effects, and the monitoring required.
Ustekinumab (Anti-12/23)
Ustekinumab is a monoclonal antibody which binds to the p40 subunit of IL-12 and IL-23 and is injected subcutaneously via a pre-filled syringe. Typical dosing for psoriasis or psoriatic arthritis is 45 mg for those weighing less than 100 kg and 90 mg for those weighing over 100 kg, administered as a loading dose at weeks 0 and 4, followed by maintenance dosing every 12 weeks after that. Ustekinumab is also used in treatment of Crohn’s disease and ulcerative colitis, using a weight-based intravenous induction dose, followed by 90 mg injected subcutaneously every 8 weeks thereafter. Patients with severe Crohn’s Disease may be dosed every 4 weeks thereafter.
Data supporting the use of ustekinumab for HS are limited to small (fewer than 20 patients), uncontrolled studies showing response to therapy by Hidradenitis Suppurativa Clinical Response (HiSCR) in roughly 50% of patients, along with improvement in measures of pain and quality of life.
Little evidence is currently available to guide the dosing regimen for ustekinumab in HS. Anecdotally, higher dose and frequency of medication administration (e.g., a maintenance regimen of 90 mg every 6 to 8 weeks) may, in the authors’ experience, be more effective. However, in general, patients with severe HS who have failed anti-TNF therapy frequently have an inadequate response to ustekinumab, regardless of dose. This option may be attractive in those patients with comorbid HS and IBD, in which case ustekinumab is reasonable for management of both diseases. The infrequency of dosing is also a relative advantage over other regimens. Medication monitoring consists of tuberculosis screening.
Guselkumab (Anti-IL-23)
Guselkumab is a monoclonal antibody which binds to the p-19 subunit of IL-23 and is delivered subcutaneously at a dose of 100 mg at weeks 0 and 4, then every 8 weeks after that. Its successful use has been reported in a handful of case reports. A recent report of four cases and literature review suggests clinical benefit is modest, with fewer than half (7/16) of patients included in the review having clinical improvement (reduction of ≧ 1 point on HS-PGA or ≧ 2 points on DLQI or VAS for pain) at week 12.
Anakinra (Anti-IL-1)
Anakinra is an injectable IL-1α and β inhibitor developed for use in patients with autoinflammatory diseases. Dosing is standardized at 100 mg administered as a once-daily subcutaneous injection. Case reports and series have suggested a beneficial effect for selected patient with HS. One small randomized, controlled trial demonstrated decreased disease activity, with 78% (7 of 9) of those receiving anakinra achieving HiSCR at 12 weeks, compared to 30% (3 of 10) in the placebo arm. Circulating interferon-γ production was also decreased in the treatment arm. Other endpoints, however, including visual analogue scale (VAS) scores, pain scores, and Sartorius scores, were unchanged in both the treatment and placebo groups following 24 weeks of therapy.
In practice, as with ustekinumab, those treated with anakinra frequently have severe HS unresponsive to TNF-inhibitors; thus, response rates may be lower than reported in the literature. Unfortunately, anakinra is not a good option for patients with comorbid IBD, since it is not an effective treatment for that indication. Compared to the dosing regimens of other biologics, the daily injections of anakinra are inconvenient and more frequently complicated by painful injection site reactions. General advice for preventing reactions includes warming the medication to room temperature before injection, cooling the injection site with an ice pack for a few minutes before and after injection, applying a topical steroid to the area, and rotating injection sites. Monitoring of the absolute neutrophil count should occur monthly for three months, then every three months for the first year. Unlike ustekinumab and the TNF inhibitors, anakinra should be adjusted for renal impairment.
Canakinumab (Anti-IL-1β)
Canakinumab is a long-acting anti-IL-1β monoclonal antibody, approved for various autoinflammatory syndromes. Unlike anakinra, the medication is typically dosed every 4 to 8 weeks subcutaneously by weight. Scant data are available, with a small number of case reports describing both response and non-response to therapy.
Secukinumab (Anti-IL-17)
Secukinumab is an anti-IL-17 agent which has been investigated for management of hidradenitis suppurativa. Standard dosing is 300 mg injected subcutaneously weekly for 5 weeks, followed by 300 mg every 4 weeks after that. An open-label study and a retrospective review reported HiSCR of 70% (14/20) and 75% (15/20), respectively, in patients with moderate to severe HS receiving secukinumab, without comparison to a control group.
Secukinumab monitoring includes screening for tuberculosis. Additionally, there is concern that use of IL-17 inhibitors may be associated with IBD. HS itself is associated with an increased prevalence of IBD. Consequently, patients treated with secukinumab should be monitored for gastrointestinal symptoms. Secukinumab is not a good choice for management of coexisting HS and IBD.
Ixekizumab (Anti-IL-17)
Ixekizumab is another anti-IL-17 agent dosed subcutaneously every 4 weeks following an initial loading dose. Only two case reports describing the use of ixekizumab in HS are available, both demonstrating improvement in HS among patients with concomitant psoriasis.
Apremilast (PDE4)
Apremilast is an oral phosphodiesterase 4 (PDE4) inhibitor which is dosed at 30 mg twice daily, following a 5-day titration period. The utility of apremilast for HS has been investigated in a case series and an open-label study, which showed modest benefit in treating HS, which was relatively less severe. A small randomized controlled trial enrolled 20 patients with moderate HS, randomized 3:1 to apremilast versus placebo. Eight of 15 (53%) treated patients met the HiSCR at week 16, versus 0 of 5 (0%) of those receiving placebo ( P = .055).
Apremilast has been associated with gastrointestinal side effects such as diarrhea and nausea, weight loss, and depression. It appears to be only modestly effective for treatment of HS, and the cost of the medication may be prohibitive if insurance coverage cannot be obtained. Nevertheless, as an oral medication with a novel mechanism of action, it may be a potentially attractive option for some patients.
Tofacitinib (JAK Inhibitor)
Tofacitinib, generally dosed at either 5 mg or 10 mg orally, twice daily 5 mg orally, twice daily, is a Janus kinase (JAK) inhibitor which acts through the JAK-STAT pathway to suppress inflammatory cytokines, including IL-1β, IL-6, and TNF-α. A report of two patients with recalcitrant, ulcerative HS described response to tofacitinib dosed at 5 mg twice daily, in concert with other therapies, where prior treatment with other biologics had failed.
Special Circumstances in the Use of Biologic Therapy for Hidradenitis Suppurativa
Pregnancy and Lactation
Not enough is known about the effects of pregnancy on HS, and vice versa. Despite being most common in women of child-bearing age, HS has not been systematically studied in the context of pregnancy and lactation.
Available data suggest HS may worsen during pregnancy in 61.9% (70/113), versus staying the same in 30.1% (34/113) or improving in 8.0% (9/113) of pregnancies; meanwhile, 66.1% (82/124) of pregnancies led to worsening HS in the postpartum period. Despite this, many HS treatment options are contraindicated in pregnancy, and the minority of patients receive HS-specific therapy during pregnancy. A registry has been created to help address knowledge gaps related to hidradenitis suppurativa and pregnancy.
For patients with moderate or severe HS who have been stably managed on a biologic therapy or who meet criteria for initiation of a biologic may, in many cases, continue these needed therapies, similar to patients with other systemic inflammatory conditions like rheumatoid arthritis or IBD. The benefits of adalimumab and infliximab are felt to outweigh the risks in the first and second trimesters. Available data suggest exposure to TNF inhibitors in utero does not increase the risk of pregnancy-related complications. During the third trimester, when the placenta is most permeable to transfer of maternal IgG antibodies, and translocation of the drug across the placenta could result in neonatal immunosuppression, the risks and benefits of anti-TNF therapy should be weighed. Among TNF inhibitors, certolizumab is considered the safest during pregnancy. As a pegylated TNF inhibitor, its larger molecular size limits placental transfer, but there are no data to support its use in hidradenitis. Ustekinumab and secukinumab may be used safely during pregnancy, but the use of anakinra and apremilast is discouraged given limited safety data.
In general, the TNF-inhibitors and ustekinumab are considered safe during lactation, whereas caution is advised for anakinra, secukinumab, and apremilast. It is always safest to review the most up-to-date information on pregnancy and lactation in a drug reference guide before prescribing. Coordination of care between dermatology and obstetrics-gynecology is essential in these complex cases. Special considerations for HS in women are further discussed in Chapter in 31 .
Pediatric Population
Direct evidence for the use of biologic therapies for management of hidradenitis suppurativa in the pediatric population is limited. However, some assumptions can be made on the basis of efficacy data from the adult population and safety data derived from the use of biologics in children with other inflammatory diseases.
In 2016, adalimumab was approved by the FDA for use in adolescents with HS (age 12 years and older) using a model-informed drug development approach, extrapolated from the phase 3 studies performed in adult patients, population pharmacokinetic simulations, and long-term safety data. As for adults, adalimumab and infliximab are recommended for pediatric patients with severe HS.
Naturally, data guiding the use of other biologic therapies in the pediatric population are fewer. Successful use of ustekinumab has been reported, and the safe long-term use of anakinra for pediatric autoinflammatory diseases is well-established. Dosing of biologic agents in children should be adjusted according to weight. Special considerations for HS in children are further discussed in Chapter in 29.
Use of Biologic Therapies in the Setting of Malignancy
Large pooled datasets looking specifically for an increased risk of lymphoma or malignancy have found no such risk in patients on long-term anti-TNF therapy as compared to patients with the same inflammatory condition on alternative drugs. A meta-analysis of patients with rheumatoid arthritis and prior malignancy found no increased risk of new or recurrent cancer among those receiving biologic therapies (including TNF inhibitors, rituximab, or anakinra) compared to other disease-modifying anti-rheumatic drugs (DMARDs). A large cohort study similarly found the risk of incident malignancy among rheumatoid arthritis patients receiving biologic therapies was no higher than for those receiving DMARDs or those in a general population comparator group. A retrospective chart review of patients with psoriasis and history of malignancy similarly found no increased risk of recurrence or progression of cancer associated with use of biologic therapies, including among a small number of patients receiving concurrent cancer therapy.
While these data are reassuring, caution is advised. In the setting of malignancy or a history of malignancy, it is important to consider carefully the risks and benefits of biologic therapy, including which biologic therapy, on an individual basis, with the help of the patient’s oncologist and primary physician. In some cases, the prospect of improved control of severe and life-altering HS will outweigh potential risks. Dermatologic input may provide key perspective in guiding this medical decision making.
Conclusion
While adalimumab may help many patients with moderate-to-severe HS, the disease is often refractory, necessitating trials of other agents. High-dose infliximab is successful in many such patients. For those who cannot tolerate or fail to respond to anti-TNF therapy, other targeted therapies should be considered, depending on patient comorbidities. Additional options include anti-IL1 therapy and anti-IL12/23 inhibitors. Anti-IL17 therapies, JAK inhibitors, and PDE4 inhibitors have also been trialed. New medications are in development to help those patients who remain refractory to existing targeted therapies. The need for expanded treatment options for moderate-to-severe HS remains significant.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree