Superficial Fungal Infection: Introduction
|
Mycoses
Mycoses are divided among three forms: (1) superficial, involving stratum corneum, hair, nails, (2) subcutaneous, involving dermis and/or subcutaneous tissue, and (3) deep/systemic, representing hematogenous spread of organisms including opportunistic pathogens in immunocompromised hosts. The focus of this chapter is the superficial mycoses and their patterns of integumentary infections (Table 188-1). A glossary of terms used in this chapter is contained in Table 188-2.
Anthropophilic—preferring humans over other animals as natural habitat Arthroconidia—asexual spore produced by segmentation of hyphae Dematiaceous—melanin in the cell walls of its conidia, hyphae, or both results in a darkly colored fungus Ectothrix—dermatophyte growth pattern with spores forming a sheath on the outside of the hair shaft Endothrix—dermatophyte growth pattern with spore formation within the hair shaft Favus—dermatophyte growth pattern with hyphae and air spaces within the hair shaft Geophilic—preferring the soil over humans and animals as natural habitat Hyphae—long, filamentous fungus cells forming a branching network called mycelium Macroconidia—asexual large multinucleate spores produced by vegetative reproduction Microconidia—asexual small spores produced by vegetative reproduction Zoophilic—preferring animals over humans as natural habitat |
Dermatophytes
The universe of fungi comprises more than 1.5 million species worldwide. Dermatophytes (term derived from the Greek words for “skin plant”) are contained in the family of arthrodermataceae and are represented by approximately 40 species divided among the three genera: Epidermophyton, Microsporum, and Trichophyton. In the United States, Trichophyton species, and namely T. rubrum and T. interdigitale, represent the most common species isolated. Dermatophytes are classified further according to their natural habitats—humans, animals, or soil. Their ability to attach to and invade keratinized tissue of animals and humans and to utilize degradation products as nutritional sources form the molecular basis for superficial fungal infections of skin, hair, and nails, termed dermatophytoses.1
Recent modifications to the taxonomical system of dermatophytes affecting clinical practice require mention. While previous taxonomy was based largely on phenotypical characteristics of dermatophytes, recent inclusion of genotypical analyses necessitated regrouping of some taxa since many of these genotypical differences were not reflected phenotypically, and vice versa.2 Current taxonomy includes a synthesis of new data based on sequencing of variable genomic regions such as the internal transcribed spacer (ITS) regions of fungal ribosomal DNA as well as classic phenotypical characterizations. The difficulty in devising such a taxonomical system for dermatophytes relates to reduced genetic diversity due to recent speciation and population of the same ecological niches. Phenotypically, this is reflected by similar clinical manifestations being caused by multiple taxonomically different dermatophyte species. It should be noted, however, that the current framework is still a work in progress, and the taxonomy will likely undergo further refinements in the future. Table 188-3 lists the most commonly encountered dermatophyte pathogens including the new taxonomy according to their natural habitats and reservoirs. The current medical literature on dermatophytes and infections, however, does not stringently follow the new taxonomy. In order to avoid confusion over the dynamic status of the taxonomy as well as to remain reflective of the current nomenclature in the literature, this chapter will use both nomenclatures, resulting in some apparent contradictions. The authors hope that a more unified nomenclature is accepted for future editions of this chapter.
Habitat | Dermatophyte | Host |
|---|---|---|
Anthropophilic | Trichophyton rubrum | Humans |
Trichophyton tonsurans | ||
Trichophyton interdigitale (syn: Trichophyton mentagrophytes var. interdigitale) | ||
Trichophyton schoenleinii | ||
T. rubrum (syn: Trichophyton megninii, Trichophyton gourvilii) | ||
Trichophyton soudanense | ||
Trichophyton violaceum (syn: Trichophyton yaoundei) | ||
Trichophyton concentricum | ||
Microsporum audouinii | ||
Microsporum ferrugineum | ||
Epidermophyton floccosum | ||
Zoophilic | T. mentagrophytes (syn: T. mentagrophytes var. quinckeanum) | Rodents |
T. interdigitale (syn: T. mentagrophytes var. mentagrophytes, T. mentagrophytes var. granulosum) | Rodents | |
Trichophyton erinacei | Hedgehogs | |
Trichophyton simii | Primates | |
Trichophyton verrucosum | Cattle | |
Microsporum canis (syn: Microsporum distortum, Microsporum equinum) | Cats, dogs, horses | |
Microsporum amazonicum | Rodents | |
Microsporum gallinae | Poultry | |
Microsporum nanum | Pigs | |
Microsporum persicolor | Rodents | |
Geophilic | Microsporum gypseum | |
Microsporum cookie | ||
Microsporum persicolor | Soil | |
Trichophyton vanbreuseghemii | ||
Trichophyton eboreum | ||
Trichophyton terrestre |
The additional classification of superficial fungi according to natural habitat is clinically relevant, since anthropophilic, zoophilic, and geophilic dermatophytoses provide important information about the source of infection and demonstrate varied clinical features.
species are typically restricted to human hosts and are transmitted via direct contact. Infected skin or hair retained in clothing, combs, caps, socks, and towels, for example, also serve as source reservoirs. Unlike the sporadic geophilic and zoophilic infections, anthropophilic infections are often epidemic in nature. These dermatophytes have adapted to humans as hosts and as such elicit a mild to noninflammatory host response.
species are transmitted to humans from animals. Cats, dogs, rabbits, guinea pigs, birds, horses, cattle and other animals are common sources of infection. Transmission may occur through direct contact with the animal itself, or indirectly via infected animal hair. Exposed areas such as the scalp, beard, face, and arms are favored sites of infection. Microsporum canis is often transmitted to humans from cats and dogs, while guinea pigs and rabbits are a frequent source of human infection with zoophilic strains of T. interdigitale. While host adaptation by zoophilic dermatophytes may lead to relatively silent infections, these dermatophytes tend to produce acute and intense inflammatory responses in humans.1
fungi cause sporadic human infection upon direct contact with the soil. Microsporum gypseum is the most common geophilic dermatophyte cultured from humans. There is a potential for epidemic spread due to the higher virulence of geophilic strains as well as an ability to form long-lived spores that may reside in blankets or grooming tools. As with zoophilic infections, geophilic dermatophytes typically result in intense inflammatory responses.3
Clinical presentations of dermatophytoses depend not only on the source, but also on host factors. Immunocompromised individuals are more susceptible to refractory dermatophyte infections or to deep mycoses.4,5 Interestingly, only the severity of dermatophytosis appears to be increased with HIV infection, and not the prevalence.6 Other host factors such as age, sex, and race appear to be additional epidemiologic factors for infection, although their relationship to dermatophyte susceptibility remains unclear. As an example, dermatophyte infections are five times more prevalent in males than females.
Superficial fungal infections are a worldwide problem that affects more than 20%–25% of the population.7 Some species demonstrate ubiquitous distribution whereas others are geographically limited. Accordingly, predominant species reflect considerable geographic differences, as in the case of tinea capitis. In the United States, Trichophyton tonsurans has replaced Microsporum audouinii as the most common cause of tinea capitis in the second half of the 20th century, and M. canis has now become the second most common cause.8 In Europe, M. canis remains the most common cause of tinea capitis despite a significantly increased incidence of T. tonsurans.9 The etiologic profile is quite different in Africa where M. audouinii, Trichophyton soudanense, and Trichophyton violaceum are the most prevalent pathogens.10 However, human travel and migration results in dynamic patterns of infection. As an example, T. soudanense and T. violaceum, typically restricted to Africa, were isolated in US cases of tinea capitis in 2007.11 Finally, local customs may also influence rates and patterns of dermatophytoses. The use of macerating occlusive footwear, for example, in industrialized nations has made tinea pedis and onychomycosis much more common in these regions.6
Dermatophytes exhibit a broad armamentarium of enzymes (keratinolytic proteases, lipases etc.) that act as virulence factors to allow adherence and invasion of skin, hair, and nails, and also to utilize keratin as a source of nutrients for survival. The initial steps in dermatophyte infections are adherence to keratin followed by invasion and growth of mycelial elements. As a consequence of keratin degradation and subsequent release of proinflammatory mediators, the host develops an inflammatory response of varying degree. The classic “ringworm,” or annular morphology of tinea corporis results from an inflammatory host response against a spreading dermatophyte followed by a reduction or clearance of fungal elements from within the plaque, and in many cases by spontaneous resolution of the infection.
Dermatophytes overcome several lines of host defense before hyphae begin to thrive in keratinized tissues. The first step is successful adherence of arthroconidia, asexual spores formed by fragmentation of hyphae, to the surface of keratinized tissues.12 Early nonspecific lines of host defense include fungistatic fatty acids in sebum as well competing bacterial colonization.13,14 Several recent studies have focused on the molecular steps involved in arthroconidial adherence to keratinized surfaces. Dermatophytes have been shown make selective use of their proteolytic armamentarium during adherence and invasion.15,16 The basis for this highly concerted attack may be explained partially by specific upregulation of multiple genes induced by contact with keratin, as has been shown by differential gene expression analysis in T. rubrum.17 Following several hours of successful adherence, the spores begin to germinate in preparation for the next step in the infective chain of events, invasion.
Trauma and maceration facilitate penetration of dermatophytes through the skin. Invasion of germinating fungal elements is further accomplished through secretion of specific proteases, lipases and ceramidases, the digestive products of which also serve as fungal nutrients.18 Interestingly mannans, which are components of the fungal cell wall, show inhibitory effects on keratinocyte proliferation and cell-mediated immunity.19,20
Dermatophytes encounter a range of host responses from several lines of nonspecific mechanisms including fungistatic fatty acids, increased epidermal proliferation, and secretion of inflammatory mediators to cell mediated-immunity. In the line of defense mechanisms, keratinocytes represent the first frontier of living cells to encounter invading fungal elements. The key position of keratinocytes is reflected by their complex response to invasion including proliferation to increase shedding as well as secretion of antimicrobial peptides including human β defensin-221 as well as proinflammatory cytokines (IFN-α, TNFα, IL-1β, 8, 16, and 17) that further activate the immune system. Once deeper layers of epidermis are involved, new nonspecific defenses such as competition for iron by unsaturated transferrin emerge. The degree of host inflammatory reaction depends on the host’s immune status as well as the natural habitat of the dermatophyte species involved. Interestingly, anthropophilic dermatophytes induce secretion of a limited cytokine profile from keratinocytes in vitro compared to zoophilic species.22,23 This difference may reflect the augmented inflammatory response generally observed with zoophilic species.
The next level of defense is cell-mediated immunity resulting in a specific delayed type hypersensitivity response against invading fungi. The inflammatory response associated with this hypersensitivity is associated with clinical resolution, while defective cell-mediated immunity may result in chronic or recurrent dermatophytoses. The Th2 response does not appear to be protective, since patients with elevated fungal antigen antibody titers are observed to have widespread dermatophyte infections.24 A possible role for the Th17 response to dermatophyte infections is suggested by the recent discovery of binding of hyphal elements to Dectin-2, a C-type lectin pattern recognition receptor on dendritic cells, critical for inducing Th17 responses.25,26 However, the relative importance of the Th17 immune response to dermatophytosis remains to be elucidated.
Despite epidemiological observations suggesting a genetic predisposition to fungal infections, molecular insight confirming this hypothesis has been lacking. Recently, however, two families with increased susceptibility to fungal infections and mutations in the C-type lectin fungal pattern recognition pathway have been described. In addition, mutations in CARD9, an adaptor molecule downstream of Dectin-1 and Dectin-2, which result in failure of Th17 activation, were associated with susceptibility to chronic mucocutaneous candidiasis along with chronic dermatophyte infections.27
Diagnostic Procedures
Laboratory Test | Method | Function | Findings |
|---|---|---|---|
Potassium hydroxide preparation | Scales from the advancing border, subungual debris, or affected hair removed and placed on a glass slide. KOH 10% dropped on specimen and covered with a cover slip. The undersurface of the glass slide may be gently heated with a low-lit flame. | KOH solution and gentle heating softens keratin and highlights the dermatophyte. | Long narrow septated and branching hyphae |
Culture | Sabouraud medium (4% peptone, 1% glucose, agar, water) | Facilitates growth of dermatophytes | Microscopic morphology of microconidia and macroconidia, along with culture features including surface topography and pigmentation. The reader is referred to http://www.mycology.adelaide.edu.au/ for a comprehensive characterization of fungal colonies. Common colonies are characterized in Table 188-5. |
Modified Sabouraud medium (addition of chloramphenicol, cycloheximide, and gentamicin) | Facilitates growth of dermatophytes and inhibits growth of non-Candida albicans, Cryptococcus, Prototheca species, P. werneckii, Scytalidium species, Ochroconis gallopava | ||
Dermatophyte test medium | Scales from the advancing border, subungual debris or affected hair embedded in the medium. | Medium contains the pH indicator phenol red. Dermatophytes utilize proteins resulting in excess ammonium ion and an alkaline environment. | Incubation at room temperature for 5–14 days results in change in color of medium from yellow to bright red in the presence of a dermatophyte. |
Histolopathology special stains: periodic acid-Schiff and Grocott’s methenamine silver | Tissue may be obtained by skin or nail biopsy techniques | Stains fungal cell wall to detect fungal elements in tissue sections | Pink (PAS) or black (GMS) fungal elements noted in the stratum corneum |
The clinical diagnosis of a dermatophyte infection can be confirmed by microscopic detection of fungal elements, by identification of the species through culture, or by histologic evidence of the presence of hyphae in the stratum corneum. In addition, fluorescence patterns under Wood’s light examination may support a clinical suspicion.
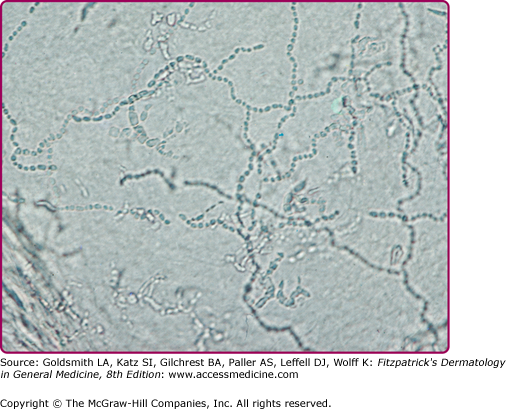
Although microscopic evaluation of potassium hydroxide (KOH)-treated samples of scale does not allow for speciation or characterization of the susceptibility profile, it is used (or underused) as a quick and inexpensive bedside tool to provide evidence of dermatophytosis. In dermatophytosis involving the skin, hair or nails, septate and branching hyphae without constriction (Fig. 188-1) may be visualized under microscopic examination with 10%–20% KOH preparation. All superficial dermatophytes appear identical when visualized in this manner. Because KOH examination may yield false-negative results in up to 15% of cases,28 patients suspected of having dermatophytosis on clinical impression should be treated. Culture confirmation should be considered whenever systemic treatment is warranted, such as in the case of tinea capitis.
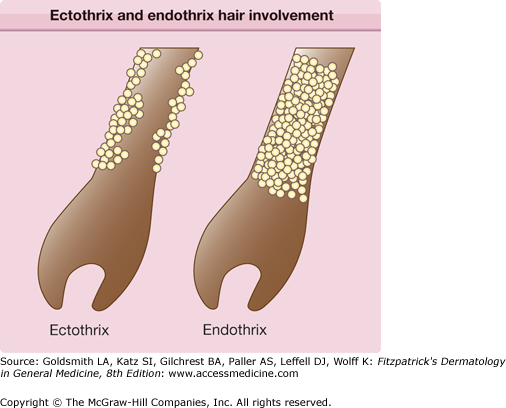
Scale from skin should be collected by scraping the involved area with a dull edge outward from the advancing margins. Full thickness nail clippings should involve the dystrophic portion, as proximal from the distal edge as possible without causing injury. Hairs should be plucked (not cut), placed on a glass slide and prepared with 10%–20% KOH and covered with a coverslip. Slightly warming the slide with a low intensity flame allows better penetration of the KOH solution into keratin. Low-power microscopy will reveal three possible patterns of infection (Fig. 188-2): (1) Ectothrix—small or large arthroconidia forming a sheath around the hair shaft, (2) Endothrix—arthroconidia within the hair shaft, or (3) Favus—hyphae and air spaces within the hair shaft.
Speciation of superficial fungi is based on macroscopic, microscopic and metabolic characteristics of the organism. While some dermatophytes are readily identified on the basis of their primary isolation cultures, most require further differentiation through subcultures on specific media (identification culture) or through specific biochemical tests.
Sabouraud’s dextrose agar (SDA) is the most commonly used isolation medium for dermatophytes and it serves as the medium on which most morphologic descriptions are based. Elimination of contaminant molds, yeast and bacteria is achieved by the addition of cycloheximide and chloramphenicol (+/−gentamicin) to the medium making it highly selective for the isolation of dermatophytes. The development of colonies can take 5–7 days in the case of Epidermophyton floccosum and up to 4 weeks for Trichophyton verrucosum. Cultures are incubated at room temperature (20°C–25°C) for at least 4 weeks before being finalized as no growth. Dermatophyte test medium (DTM) is an alternative isolation medium that contains the pH indicator phenol red. The medium turns red when dermatophyte proteolytic activity increases the pH to 8 or above, and it remains amber with the growth of most saprophytes. Nondermatophyte acidic byproducts turn the medium yellow. While DTM serves as a good alternative for isolation of dermatophytes, it may not allow for their direct identification due to altered growth and thus morphology of dermatophytes in DTM. Table 188-5 describes general microscopic features of microconidia and macroconidia of the three genera of dermatophytes, while Table 188-6 describes colony and microscopic features of the most common dermatophyte species.
Genera | Microconidia | Macroconidia |
|---|---|---|
Trichophyton | Smooth walled. Used for identification. | Absent or nondiagnostic. |
Microsporum | Absent or nondiagnostic. | Rough walled. Used for identification. |
Epidermophyton | Absent. | Smooth walled. Used for identification. |
Organism | Colony Morphology | Microscopic Appearance | |
|---|---|---|---|
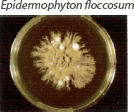 | Flat feathery colonies with a central fold and yellow to dull gray–green pigment. Yellow to brown reverse pigment. | 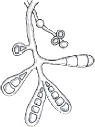 | Numerous thin and thick-walled, club-shaped macroconidia. |
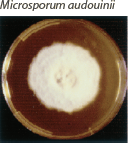 | Flat and white to gray with widely spaced radial grooves. Tan to salmon reverse pigment. Salmon-pink pigment on PDA. No growth on polished rice. | 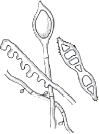 | Terminal chlamydoconidia and pectinate (comb-like) hyphae. |
 | Flat, white to light yellow, coarsely hairy, with closely spaced radial grooves. Yellow to orange reverse pigment. Yellow on PDA. Growth on polished rice. | 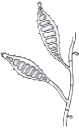 | Numerous thick walled and echinulate spindle shaped macroconidia with terminal knobs and greater than 6 cells. |
 | Flat and granular with tan to buff pigment, no reverse pigment. | 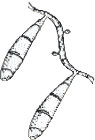 | Numerous thin-walled pickle shaped macroconidia without knobs and fewer than 6 cells. |
 | White to creamy with a cottony, mounded surface. None to light brown reverse pigment. No pigment on PDA. Urease positive, which helps to distinguish it from T. rubrum. | 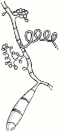 | Grape-like clusters of round microconidia, rare cigar-shaped macroconidia, occasional spiral hyphae. Hair perforation positive, which helps to distinguish it from T. rubrum. |
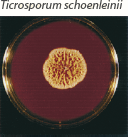
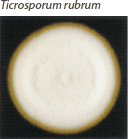 | Mounded white center with maroon periphery. Maroon reverse pigment. Cherry red on PDA. Urease negative. | 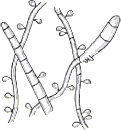 | Few tear-shaped microconidia, rare pencil-shaped macroconidia. Hair perforation negative. |
 | Heaped or folded and whitish. Colorless to yellow-tan reverse pigment. | 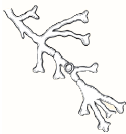 | Knobby antler-like hyphae (favic chandeliers), numerous chlamydoconidia. |
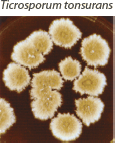 | Suede-like center with feathery periphery, white to yellow or maroon color. Reverse pigment usually dark maroon, sometimes none to yellow. Partial thiamine requirement. | 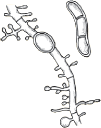 | Numerous multiform microconidia and rare cigar-shaped macroconidia. |
 | Small and heaped, although sometimes flat, white to yellow–gray. Reverse pigment none to yellow. Requires thiamine and usually inositol for growth. |  | Chains of chlamydoconidia on SDA. Long and thin “rat-tail” macroconidia with thiamine. |
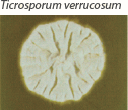
 | Waxy and heaped, deep purplish-red. Purple reverse pigment. Partial thiamine requirement. | 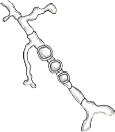 | Irregular hyphae with intercalary chlamydoconidia. No micro- or macroconidia on SDA, rare micro- and macroconidia with thiamine. |
PDA = potato dextrose agar; SDA = Sabouraud’s dextrose agar. Used with permission from David Ellis, PhD. | |||
Identification of isolated fungi is facilitated by subculture on specific media such as potato dextrose agar (PDA) or Borelli’s lactrimel agar (BLA) that stimulate sporulation, production of pigment and development of typical morphology. Finally, dermatophytes may be differentiated further by their ability to grow on autoclaved polished rice, perforate short strands of hair in vitro or hydrolyze urea (urease test), or require nutritional supplementation for growth (Table 188-7).
Urease test | Differentiates Trichophyton. interdigitale (positive result) from Trichophyton rubrum (negative result) | |
Hair perforation test | Differentiates T. interdigitale (positive result) from T. rubrum (negative result) | |
Nutritional requirement | Differentiates Trichophyton species | |
Trichophyton tonsurans | ||
Thiamine | Trichophyton concentricum | |
Trichophyton violaceum | ||
Thiamine + inositol | Trichophyton verrucosum | |
Nicotinic acid | Trichophyton equinum | |
Histidine | Trichophyton megninii | |
Growth on polished rice | Differentiates Microsporum species | |
Good growth | Mrichophyton canis | |
Poor growth | Mrichophyton audouinii | |
Mrichophyton distortum | ||
Skin biopsy is not often employed in the workup of typical dermatophytoses. Localized cutaneous eruptions suspected to represent dermatophytosis with equivocal KOH examination are often treated despite the lack of confirmation. Biopsy may confirm the diagnosis when a systemic agent is being considered for treatment of a recalcitrant or more widespread eruption. Biopsy may be used to aid in the diagnosis of Majocchi’s granuloma in which KOH examination of scale on the surface may more often be negative. Biopsy is also sometimes useful in confirming the presence of hyphae involving hair shafts on the scalp in tinea capitis, although culture is necessary to allow speciation of the pathogen. When present, hyphae may be appreciated in the stratum corneum on hematoxylin and eosin staining. However special stains, most commonly periodic acid-Schiff (PAS) and methenamine silver stains, highlight hyphae that may otherwise be subtle in appearance on routine staining. Whereas culture is the most specific test for onychomycosis, PAS examination of nail clippings is the most sensitive29 and it obviates the need to wait weeks for a result.
Examination of involved hair bearing areas, such as the scalp or beard, with a Wood’s lamp (365 nm) may reveal pteridine fluorescence of hair infected with particular fungal pathogens. Hairs that fluoresce should be selected for further examination, including culture. While ectothrix organisms M. canis and M. audouinii will fluoresce on Wood’s light examination, the endothrix organism T. tonsurans will not fluoresce. T. tonsurans, which is now the most common cause of tinea capitis in the United States, thus limits the use of Wood’s light examination. Table 188-8 lists common patterns of dermatophyte hair involvement and fluorescence.
Pattern | Dermatophyte | Fluorescence |
|---|---|---|
Endothrix | Trichophyton soudanense | None |
Trichophyton violaceum | None | |
Trichophyton tonsurans | None | |
Trichophyton gourvilii | None | |
Trichophyton yaoundei | None | |
Ectothrix | Mrichophyton canis | Yellow–green |
Mrichophyton audouinii | Yellow–green | |
Mrichophyton distortum | Yellow–green | |
Mrichophyton ferrugineum | Yellow–green | |
Mrichophyton fulvum | None | |
Mrichophyton gypseum | None | |
Trichophyton megninii | None | |
Trichophyton interdigitale | None | |
Trichophyton rubrum | None | |
Trichophyton verrucosum | None | |
Favus | Trichophyton schoenleinii | Blue–gray, occasional |
Dermatophytoses
Tinea capitis describes dermatophyte infection of hair and scalp, typically caused by Trichophyton and Microsporum species, with exception of Trichophyton concentricum.
Tinea capitis is most commonly observed in children between 3 and 14 years of age. The fungistatic effect of fatty acids in sebum may help to explain the sharp decrease in incidence after puberty.30 Overall prevalence of the carrier state is around 4% in the United States with a peak prevalence of approximately 13% in girls of Sub-Saharan African American descent.31 In general, tinea capitis is more common among children of African descent for unknown reasons. Transmission is increased with decreased personal hygiene, overcrowding and low socioeconomic status. The anthropophilic dermatophyte T. tonsurans is the most prevalent species found in the United States, while M. canis remains the most common cause of tinea capitis in Europe.32 Organisms responsible for tinea capitis have been cultured from fomites such as combs, caps, pillowcases, toys and theater seats. Even after shedding, hairs may harbor infectious organisms for more than 1 year.33 The high prevalence of asymptomatic carriers thwarts eradication of the disease.
Infection of hair by dermatophytes follows 3 main patterns—ectothrix, endothrix and favus. Dermatophytes establish infection in the perifollicular stratum corneum and spread around and into the hair shaft of mid- to late-anagen hairs before descending into the follicle to penetrate the cortex. With hair growth, the infected part of the hair rises above the surface of the scalp where it may break because of its increased fragility.
In ectothrix infections (see Fig. 188-2), only the arthroconidia on the surface of the hair shaft may be visualized, although hyphae are also present within the hair shaft. The cuticle is destroyed. On Wood’s lamp examination, a yellow–green fluorescence may be detected, depending on the causative organism. In endothrix infections (see Fig. 188-2), arthroconidia and hyphae remain within the hair shaft and leave the cortex and cuticle intact. This pattern of tinea capitis is associated with the appearance of “black dots” which represent broken hairs at the surface of the scalp. Endothrix organisms do not show fluorescence on Woods lamp exam. Favus is characterized by longitudinally arranged hyphae and air spaces within the hair shaft. Arthroconidia are not usually noted in infected hairs.
(Table 188-9).
Inflammatory | Microsporum audouinii |
Microsporum canis | |
Microsporum gypseum | |
Microsporum nanum | |
Trichophyton interdigitale | |
Trichophyton schoenleinii | |
Trichophyton tonsurans | |
Trichophyton verrucosum | |
Noninflammatory | M. audouinii |
M. canis | |
Microsporum ferrugineum | |
T. tonsurans | |
Black dot | T. tonsurans |
T. violaceum | |
Favus | T. schoenleinii |
Trichophyton violaceum | |
Trichophyton mentagrophytes |
The clinical appearance of tinea capitis depends on the causative species as well as other factors such as the host immune response. In general, dermatophyte infection of the scalp results in hair loss, scaling and varying degrees of an inflammatory response.
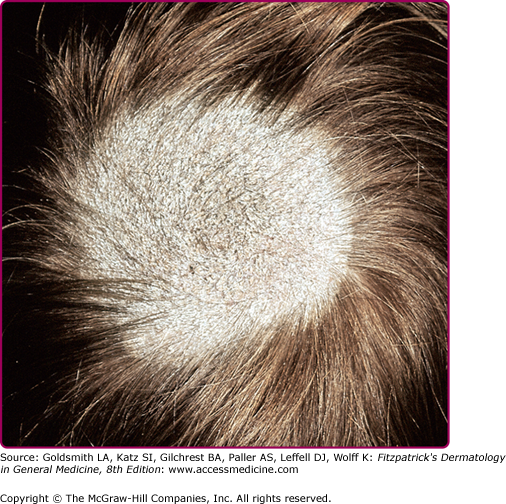
Also called the seborrheic form of tinea capitis since scale is the predominant feature,34 noninflammatory tinea capitis is seen most commonly with anthropophilic organisms such as M. audouinii or Microsporum ferrugineum. Arthroconidia may form a sheath around affected hairs turning them gray and causing them to break off just above the level of the scalp. Alopecia may be imperceptible or in more inflammatory cases there may be circumscribed erythematous scaly patches of nonscarring alopecia with breakage of hairs (“gray patch” type; Fig. 188-3). Patches often occur on the occiput.33 When involving an ectothrix pattern, infected hairs may exhibit green fluorescence under Wood’s light (see Table 188-8).
Figure 188-3
Tinea capitis “gray patch” type. A large, round hyperkeratotic plaque of alopecia due to breaking off of hair shafts close to the surface, giving the appearance of a mowed wheat field on the scalp of a child. Remaining hair shafts and scales exhibit a green fluorescence when examined with a Wood’s lamp. Microsporum canis was isolated on culture.
(Fig. 188-4).
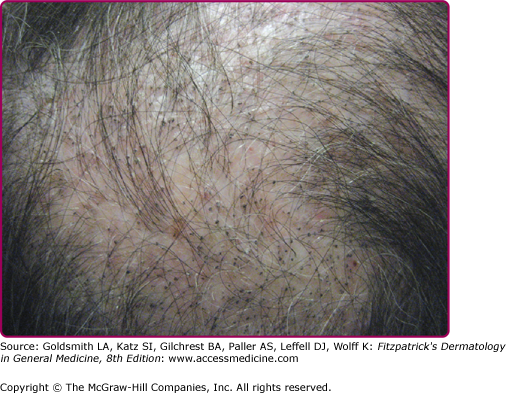
The “black dot” form of tinea capitis is typically caused by the anthropophilic endothrix organisms T. tonsurans and T. violaceum. Hairs broken off at the level of the scalp leave behind grouped black dots within patches of polygonal shaped alopecia with finger-like margins. Normal hairs also remain within patches of broken hairs. Diffuse scaling is also often present. While “black dot” tinea capitis tends to be minimally inflammatory, some patients may develop follicular pustules, furuncle-like nodules, or in rare cases kerion—a boggy, inflammatory mass studded with broken hairs and follicular orifices oozing with pus.35
Zoophilic or geophilic pathogens, such as M. canis, M. gypseum, and T. verrucosum, are more like to cause an inflammatory type of tinea capitis. Inflammation, which is the result of a hypersensitivity reaction to the infection, in this setting ranges from follicular pustules to furunculosis (Fig. 188-5) or kerion (Fig. 188-6). Intense inflammation may also result in scarring alopecia. The scalp is usually pruritic or tender. Inflammatory tinea capitis is often associated with posterior cervical lymphadenopathy, which serves as a clinical pearl in differentiating tinea capitis from other inflammatory disorders involving the scalp.