Fig. 11.1
Microscopic view of white fat tissue. Adipocytes are unilocular, separated by septa of connective tissue, richly vascularized, and innervated
Fat tissue has a mechanic function supporting organs and providing an antishock layer between the skin and underlying parts. It has also a thermoisolating function and represents the principal energetic storage under the form of lipids, stored in the adipocytes (diameter from 20 to 200 μm). The metabolic function of fat tissue is demonstrated by its ability to participate in the energetic homeostasis with its two basic processes of lipogenesis and lipolysis. Besides the lipids processed in the adipocytes, the latter secrete various peptides called adipokines, which act in an autocrine, paracrine, or endocrine way. Thus, fat tissue was shown to also have endocrine and paracrine functions, which play a role in metabolic, neuroendocrine, immune, and cardiovascular regulation (Fig. 11.2). A small part of the secreted adipokines is specific for the fat tissue like adiponectin and leptin. The majority of secreted adipokines are not specific for the fat tissue. These adipokines are: proteins (adiponectin, leptin, proteins of the complement), prostaglandins (PGE2, PGD2), growth factors (TGF-b, IGF-I, MCS-F, VEGF), interleukins (interleukin 6 (IL-6)), vasoactive substances (angiotensinogen, angiotensin, resistin), tumor necrosis factor-α (TNFα), and hepatocyte growth factor (HGF) [2–5].
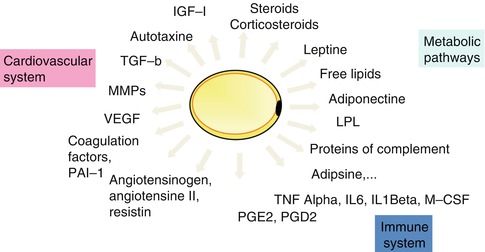

Fig. 11.2
Principles secretions of adipose tissue
Furthermore, fat tissue can participate in transforming sexual hormones, which explains the “masculinization” of obese women.
Today fat tissue is known to be the largest source of multipotent stem cells, which represent a homogeneous population of fusiform cells. These are the ASC, which have the important property of cell plasticity. This is reflected by their capacity to differentiate into adipocytes, chondrocytes, myocytes, osteocytes, neurons, hepatocytes, and endothelial cells under the appropriate conditions (Fig. 11.3) [1, 2].
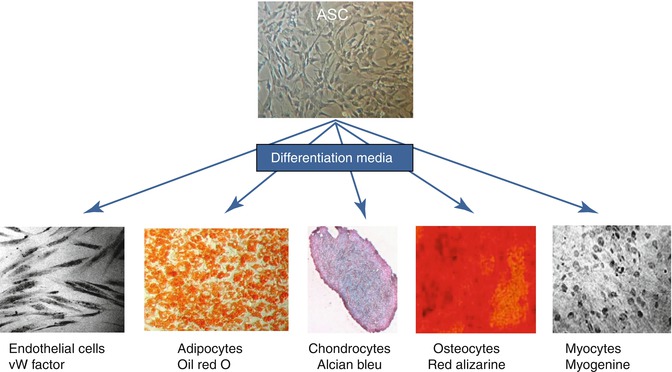

Fig. 11.3
Plasticity of ASC
To isolate ASC, it is mandatory to isolate first the SVF using collagenase treatment and centrifugation. Then, the SVF has to be plated in a specific media to select ACS after adherence to plastic dishes. ASCs are recognized using surface markers. There are no specific markers for ASC but for mesenchymal stem cells in general and these markers can be modified in culture [6].
With the discovery of ASC [7] and the fat graft regenerative effects [8], the debate has evolved into an inquiry about the role of ASC, their survival, and their involvement in regenerative programs. Obviously, the role of ASC is more complex than their simple differentiation into adipocytes. These complex relationships between ASC and all external factors physiologic (before transplantation) and ectopic (after transplantation) are known as the “niche” [9]. In biology “the niche” represents the full range of relationships that a given species establishes with the surrounding biologic, chemical, and physical environment, which directs survival and reproduction. At cellular and molecular level, this is the microenvironment of the cells (the niche). This includes the cells, matrix, and interactions that involve the physical communications between cells and cells and extracellular matrix and/or circulating or paracrine factors released by distant or local cells. We talk about cell-cell, cell-matrix relationships and paracrine responses. The exact role of all these factors that undoubtedly direct cell behavior is still poorly understood [9].
Thus, the phenotypic identity of any single cell and its reaction to a given stimulus are not determined only by the genetic equipment of the cell but also strongly dependent on the specific niche context.
11.2 Volumetric Effect: Indications and Relationship Between Components and Fat Graft Survival (Experimental Data)
The volumetric effect is used to repair missing or inadequate relief, fill depressions, reshape a previously repaired face, and improve symmetry by concealing a nonfunctional bone deficit and also reshaping an age-related deflation (Fig. 11.4) [10, 11].
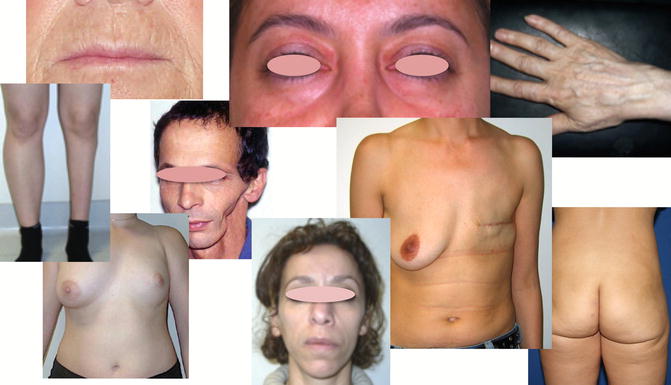

Fig. 11.4
Various indications of fat grafting – aesthetic, lower limb atrophy, head and neck cancer, breast reconstruction
Since the first fat graft, carried out at the end of the nineteenth century, the indications of fat grafting have evolved. For almost 100 years, fat has been used as soft tissue filler for volume restoration. During this past decade, fat grafting has gained increasing popularity [3].
Although the survival of adipose tissue has already been documented in different ways, including metabolic and radiological methods [2, 3, 11–13], the volumetric effect of fat grafts is concerned by a major drawback – the unpredictable resorption rates [14, 15]. Until the discovery of ASC, the favorable outcome of lipotransplants was simply attributed to the capacity of fat cells to survive. The debate was whether the success of a fat graft is the result of graft adipocyte survival (cell survival theory) or the recruitment of host adipocytes (host replacement theory) described by Peer [16, 17].
Concerning the cell source, mature adipocytes were shown to be susceptible to damage during aspiration procedures and to have limited capacity for proliferation due to their differentiated state [18]. Efforts to augment fat graft survival were made concerning fat tissue harvesting, processing, and transfer techniques [19, 20]. Although considerable knowledge was collected concerning these 3 steps (harvesting, processing, and transfer), fat graft survival rates did not reach the desired levels.
It was the discovery and more profound study of ASC that promulgated various techniques including fat grafts combined with ASC in search of improved fat graft survival. Thus, Yoshimura et al. [21, 22] proposed the cell-assisted lipotransfer. The aim was to decrease fat graft resorption by adding ASC to the fat graft. The ASC would differentiate into mature adipocytes, stimulate in vivo regeneration, and promote vascularization, which is essential for fat graft take. Thus, multipotent ASCs were introduced as an alternative source of adipose cells with high proliferation capacity and adipogenic potential. However, results remain contradictory and further investigations are required. To increase fat graft survival, autologous transplantation and adipose tissue engineering are investigated [23, 24].
Based on our experimental animal model, we tried to define which component of adipose tissue contributes to its survival to concerning the volumetric effect [25].
11.3 What Is the Contribution of Each Component for the Volumetric Effect of Fat Grafts?
To better understand the role of each component (adipocytes, ASC, and extracellular matrix) for the volume preservation after fat grafting, the authors did an experimental study in the nude mouse [25]. First, 30 mL of fat tissue were harvested 15 days before grafting, and then 60 mL were harvested on the day of grafting (quantities after centrifugation in the operating room). The first portion of adipose tissue was used for ASC isolation and culture. The cultured ASC would be seeded on collagen scaffolds (preparations 3, 5, and 6). The second portion of fat tissue (60 mL harvested on the day of grafting) was processed, as follows. The first 30 mL were injected as a fat graft (preparation 1). The second 30 mL were sent to the laboratory and used for preparation of free mature adipocytes (preparation 2) and fresh ASCs (to be seeded on collagen scaffolds, preparation 7).
After preparation, the following 7 preparations were grafted into the mice (Figs. 11.5 and 11.6): (1) purified adipose tissue (i.e., fat grafts); (2) isolated mature adipocytes without collagen scaffold; (3) cultured ASCs (passage 2) without collagen scaffold; (4) collagen scaffold only (i.e., negative control); (5) cultured ASC (passage 2) without growth factors in collagen scaffold; (6) cultured ASC (passage 2) with growth factors in collagen scaffold; and (7) freshly isolated ASCs in collagen scaffold.
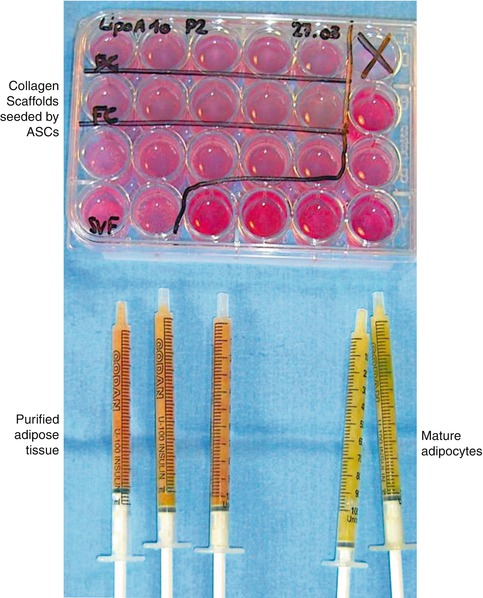
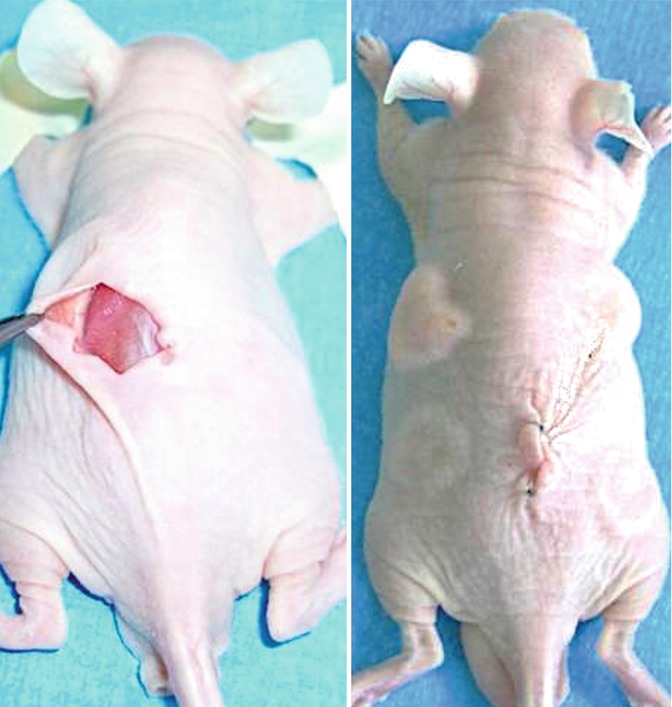

Fig. 11.5
Preparations of free-cell grafts and seeded collagen scaffolds. (Bottom) Fat graft and fresh ASC in syringes ready to be injected. (Top) Wells containing seeded collagen scaffolds to be implanted

Fig. 11.6
(Left) Implantation and injection of the studied preparations. Subcutaneous dissection via dorsal skin incision in the nude mice. (Right) The scaffolds were inserted through a single dorsal incision (the sutured incision is visible in the middle of the mouse back). Fat tissue and free-cell grafts were injected. Note the four tumefactions on the right and left sides. The upper left tumefaction represents injected fat tissue
After 2 months of implantation, mice were euthanized and the assessment was done on the following criteria:
1.
Macroscopic evaluation – observations were recorded and photographed.
2.
Mouse and implant weight – mice were weighed before and after implantation as well as before and after explantation. The individual and average weights for each group were recorded. The implants and scaffolds were weighed before implantation and after explantation.
3.
Histology and Immunohistochemistry – on the average, 20 microtome sections per graft from the periphery and the center of the samples were included ans stained with Hemotoxylin-Phloxine-Saffron. Hematoxylin stained the cellular nucleus blue, whereas phloxine stained the cytoplasm pink, and saffron stained the connective tissue orange.
4.
Human Antivimentin Antibody – vimentin, known as intermediate filament polypeptide, typically is present in cells of mesenchymal origin. This fact was used to evaluate the presence and evolution of the human ASC because these are cells of mesenchymal origin. Labeled with antihuman antivimentin antibodies, only the mesenchymal cells of human origin were stained. In this way, proliferation of mesenchymal cells of murine origin could be excluded.
5.
Oil-Red-O Staining – Oil Red O is adipocyte specific and stains lipid-filled vacuoles red.
6.
Phenotype Analysis – phenotype analysis was performed by flow cytometry as previously described for CD14 CD45, CD73, CD90, CD105, HLA DR, and HLA ABC.The following observation could be made:
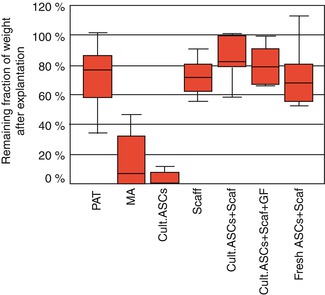
The average mouse weight increased from 26.5 to 33.7 g (±7.2 g).
11.3.1 Purified Adipose Tissue
The median weight of the explanted adipose tissue was 0.205 g, and the remaining weight fraction was 81.8 %. The grossly explanted fat was homogeneous and yellow with well-visible, abundant, newly formed vessels (Fig. 11.7).
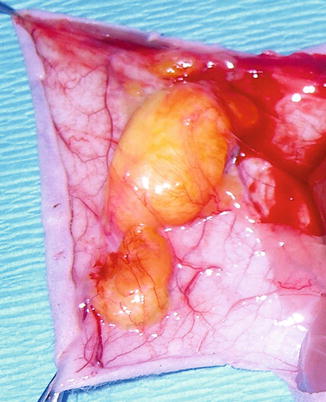

Fig. 11.7
Purified adipose tissue at explantation. Abundance of newly formed vessels around the fat tissue can be observed
Histologically, this tissue presented the 3D structure of normal adipose tissue. Both Oil-Red-O and HPS stains confirmed the presence of large adipocytes. Abundant extracellular matrix (colored orange) surrounded the adipocytes. The antihuman vimentin labeling was negative, suggesting the host origin of this matrix (Fig. 11.8).
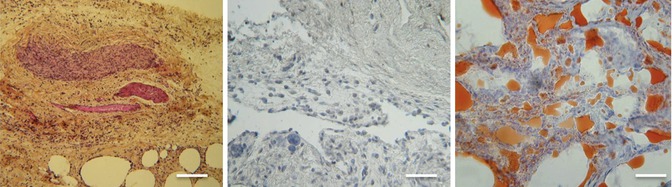

Fig. 11.8
Purified adipose tissue at explantation (microscopic view; scale, 200 lm). (Left) Both hematoxylin-phloxine-saffron (HPS) and (Right) Oil-Red-O staining confirmed the presence of large adipocytes. (Middle) The negative antivimentin staining suggests that precursor cells were transformed into mature adipocytes
11.3.2 Mature Adipocytes
Five specimens (50 %) were completely resorbed. The median weight of specimens after explantation was 0.040 g, and the remaining weight fraction was 22.5 %. In the five cases of nonresorbed adipocytes, the explanted tissue was scarce and fibrous. Oil-Red-O staining showed a small number of lipid vacuoles, and HPS staining showed no newly formed adipose tissue. Newly formed vessels were scarce.
11.3.3 Cultured ASCs Without a Scaffold
The median weight of the specimens after explantation was 0.011 g, and the remaining weight fraction was 5.3 %. Complete resorption of the graft was found in six cases (60 %). When tissue was present, it was fibrous with a small number of adipocytes (Oil Red O) and newly formed vessels.
11.3.4 Negative Control Scaffold
The median weight after explantation was 0.089 g, and the remaining weight fraction was 72.2 %. The control scaffolds appeared white and nonvascularized, without any macroscopic evidence of newly formed adipose tissue (Fig. 11.9). Histologic examination (Oil-Red-O and HPS staining) showed very few cells, and the antivimentin labeling was negative, suggesting that the few cells present were colonizers from the host.
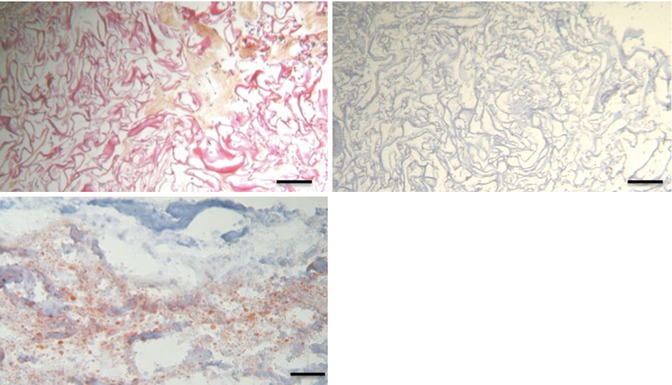

Fig. 11.9
Unseeded collagen scaffold after explantation (hematoxylin-phloxine-saffron [HPS] staining; scale, 200 lm). Note the collagen matrix (stained in red) with empty spaces and few cells
11.3.5 Scaffold Seeded with Cultured ASCs Without Growth Factors
The median weights after explantation were 0.108 and 0.097 g, respectively, and the remaining weight fractions were, respectively, 87.3 and 79.1 %. The explanted specimens showed a macroscopic view of normal adipose tissue (Fig. 11.10), with both central and peripheral newly formed vessels (angiogenesis).
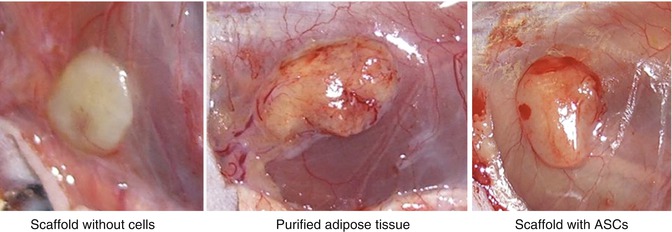

Fig. 11.10
A macroscopic view of an explanted specimen showing normal adipose tissue with both central and peripheral newly formed vessels (angiogenesis)
The newly formed tissue resembled grossly mature human fat tissue. Histologic examination showed complete settlement of the scaffold with abundant newly formed extracellular matrix and abundant mature adipocytes of human origin (Fig. 11.11).
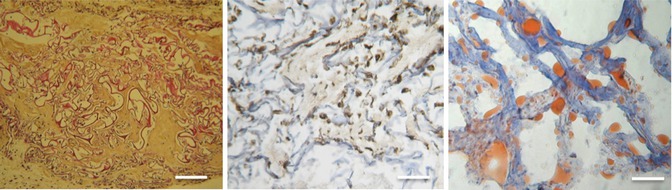

Fig. 11.11
Scaffold seeded with adipose-derived stem cells (ASCs) that have no bioactive factors after explantation (microscopic view; scale, 200 lm). (Left) Hematoxylin-phloxine-saffron (HPS) staining shows that spaces in the collagen scaffold (stained in red) are filled with connective tissue (stained in orange) that has numerous cells (stained in blue). (Middle) The antivimentin shows abundant cells of human origin (middle, cells are brown stained). (Right) Oil-Red-O staining confirms the presence of mature adipocytes via the red-stained lipid vacuoles
11.3.6 Scaffold Seeded with Cultured ASCs with Growth Factors
Ten mice received this type of graft. Preimplantation weight of grafts was 0.123 g. The median weight after explantation was 0.097 g. HPS staining revealed cells throughout the scaffolds. Human antivimentin labeling showed cells of human origin and Oil-Red-O staining revealed the presence of mature adipocytes (Fig. 11.12). In 7 of the 10 samples, both central and peripheral neovasculogenesis was noted. Additionally, Oil-Red-O staining revealed that 7 of 10 scaffolds were populated with mature adipocytes.
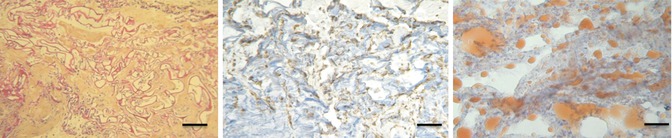

Fig. 11.12
Scaffold seeded with ASCs that have bioactive factors after explantation (scale, 200 lm). (Left) HPS staining shows cells throughout the scaffolds (stained in blue). The spaces in the collagen scaffold (stained in red) are filled with abundant connective tissue (stained in orange). (Middle) Human antivimentin labeling shows cells of human origin (cells stained in brown), and (Right) Oil-Red-O stain shows the presence of mature adipocytes (red staining)
11.3.7 Scaffold Seeded with Freshly Isolated ASCs (Stromal Vascular Fraction)
The median weight after explantation was 0.087 g. The remaining weight fraction was 70.4 %. Although the cells settled all areas of the scaffolds (HPS staining), these cells were markedly fewer compared with the other ASC-seeded scaffolds. Extracellular matrix also was less abundant with mature adipocytes in smaller numbers than in the other ASC/scaffold groups (Fig. 11.13).
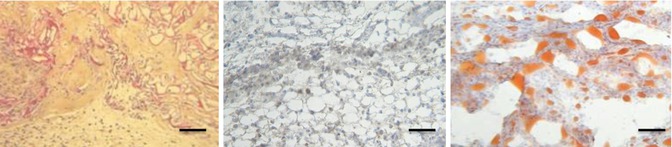

Fig. 11.13
Scaffold seeded with fresh ASCs after explantation (microscopic view; scale, 200 lm). (Left) HPS staining shows cells throughout the scaffolds. (Middle) Human antivimentin labeling shows cells of human origin (cells in brown), and (Right) Oil-Red-O stain shows the presence of mature adipocytes (red staining)
11.3.8 Statistical Analysis
The mean weight fraction of free-cell grafts (mature adipocytes and cultured ASCs) was significantly smaller compared with the other groups (p\0.05, sign test). There was a tendency toward higher weight loss in cultured ASCs than in mature adipocytes (p = 0.06, sign test) (Fig. 11.14). No significant difference was found between the other groups.