Standard Deceased Donor Kidney Procurement
Christie W. Gooden
DEFINITIONS
In the case of deceased donor kidney procurements, often the surgeon who will be transplanting the organ is not the surgeon who is procuring the organ. Therefore, it is incumbent on the procuring surgeon to take responsibility and ensure transplantability of the organ they are charged to obtain. This chapter is meant to facilitate an error-free procurement.
Deceased donors can be categorized as:
Donation after brain death: Donor has been clinically declared brain dead. Physiologic stability is usually maintained by medical measures (i.e., mechanical ventilation and vasopressors). Procuring these types of donors is what this chapter will focus on. Some also call it death by neurologic criteria.
Table 1: Definition of Standard and Expanded Criteria
Donor Condition
Donor Age Categories
<10 y
10-39 y
40-49 y
50-59 y
≥60 y
CVA + HTN + Creat >1.5
X
X
CVA + HTN
X
X
CVA + Creat >1.5
X
X
HTN + Creat >1.5
X
X
CVA
X
HTN
X
Creatinine >1.5
X
None of the above
X
X, meets definition for Expanded Criteria Donor; CVA, CVA was cause of death; HTN, history of hypertension at any time; Creat >1.5 = creatinine >1.5 mg/dL.
U.S. Department of Health and Human Services. OPTN Policy 7: allocation of deceased kidneys. http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_7.pdf-application/pdf.
Donation after cardiac death: Donor who is not brain dead has nonsurvivable injuries. Cardiac function ceases prior to organ procurement.
DONOR HISTORY
Donors are not only characterized by their manner of death. They are further distinguished by their medical history. To the procuring surgeon, the most relevant distinction is standard criteria donor versus extended criteria donor (SCD vs. ECD). ECD procurements often require the surgeon to perform additional tasks.
Standard criteria donor: Brain dead donors who do not meet any of the criteria for an ECD.
Expanded criteria donor: Donors with a medical history that is positive for predefined variables that indicate an increased risk of graft failure by 70% (relative hazard ratio, 1.70) compared with an SCD kidney (Table 1).1 Donor surgeons are often asked to biopsy these kidneys and sometimes place them on pump.
SURGICAL MANAGEMENT
Preoperative Planning
Check the consent.
Check for appropriate documentation of brain death and be aware of the clinical history and testing.
Verify that the donor name and United Network for Organ Sharing (UNOS) number match.
Positioning
Check the donor’s name with donor’s ID and conduct a “time-out” with all members of the operative team.
Position the donor supine with arms securely tucked. Assure that the anesthesia team has access to all monitors and intravascular catheters. Make sure they are working properly after the donor has been positioned.
The donor should be prepped from chin to pubis.
TECHNIQUES
EXPOSURE OF AORTA AND INFERIOR VENA CAVA
Make a midline laparotomy and sternotomy.
Perform a Cattell-Braasch maneuver to expose the inferior vena cava (IVC). This dissection should be carried from the reflection of the right colon continued superiorly to mobilize the duodenum (Kocher maneuver) and laterally to the inferior mesenteric vein (IMV) at the ligament of Treitz.
Take care to preserve the IMV, as some centers cannulate this for a portal flush (see Liver Procurement).
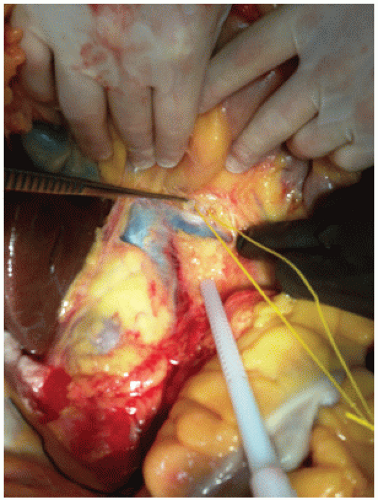
Place intestines in a sterile towel creating a “bowel bag” (FIG 1) and have the assistant reflect the bowel up and to the donor’s left. This facilitates exposure of the left and right renal veins. Dissect the fine tissue on top of the IVC to expose the left renal vein (FIG 2A). Then carefully dissect along the anterior lateral IVC to identify the right renal vein. It is important to identify both renal veins to determine where to transect the IVC later in the operation (FIG 2B).
The superior mesenteric artery (SMA) is anterior to the left renal vein.
Optional maneuver: Identify and mark SMA (FIG 3).
Using a right angle, carefully dissect the often dense fibrous or nerve tissue surrounding the SMA until the right angle can be passed around it. Take care when encircling the SMA, as the celiac artery can be close behind. Once around the SMA, pass an umbilical tape or vessel loop. The SMA is a landmark for the aortic transection later in the case. Again, this step is optional (although it is a great teaching maneuver). If the dissection proves to be difficult, the author will often abandon this maneuver as one can inadvertently injure the celiac or cause bleeding at an inopportune time.
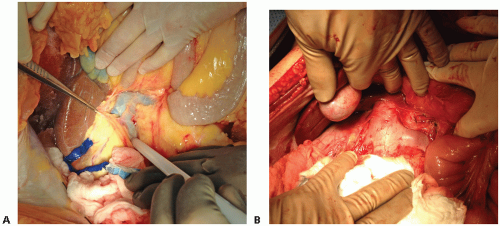 FIG 2 • A. Dissection of the tissue anterior to the IVC (indicated by the white probe) and identification of the right renal vein. B. Close-up of the renal veins. |
PREPARE INFRAHEPATIC AORTA FOR CANNULATION
Palpate the aorta next to the IVC. It is often covered by a layer of adipose and connective tissue. In an obese donor, this layer can be thick. Feel for the bifurcation and start to dissect the overlying tissue just proximal to the bifurcation in order to expose the anterior surface of the aorta. Dissect distally to expose the iliac bifurcation (FIG 4). Look for additional renal arteries when exposing the iliacs. They are usually noted on the lateral side of the common iliac artery. These are rare but important to preserve.
Circumferentially, dissect the aorta just proximal to the iliacs and mark with a heavy silk. During this dissection, be aware of lumbar branches; they are usually paired.
They can either be tied off (either ligated or just clipped so that they do not cause back-bleeding during the aortic cannulation) or avoided. The author often ignores them as they are easily dealt with once cold perfusion starts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree