, Aleksandra Klimczak2 and Grzegorz Brzezicki3
(1)
Department of Plastic Surgery, Cleveland Clinic, Cleveland, OH, USA
(2)
Department of Clinical Immunology, L. Hirszfeld Institute of Immunology and Experimental Therapy Polish Academy of Sciences, Wroclaw, Poland
(3)
Department of Neurosurgery, Virginia Commonwealth University, Richmon, VA, USA
Abstract
The advent of rapidly accelerated experience in experimental spleen transplantation models gives a hope for discovery the “Holly Grail” of transplantation tolerance. Successful spleen allotransplantations in rodents can lead to specific donor tolerance induction. Previous studies on large animal model without knowledge of major histocompatibility complex jeopardized on extinction of this idea. The reprisal of spleen allotransplantation was triggered by rapid immunology development. In this chapter we tried to describe methods of spleen transplantation and explain the new approach of spleen allograft tolerogenic effect.
Keywords
Spleen transplantationSpleen toleranceAllotransplantationSpleen immunologyIntroduction
The constant investigation of methods for allograft survival prolongation avoiding complication remains a major focus of transplantation researches. Despite many promising experimental successes in induction of tolerance as multiple bone marrow transplant procedures or different cell therapies, the clinical application is still indeterminable [1]. A solid organ transplantation technique for allograft survival prolongation has emerged again after the renaissance in 1970s. Spleen is the largest single secondary lymphoid organ and is a vital site of reticuloendothelial system [2]. This clinical dispensable organ plays an important role in both adaptive and innate immune response. Numerous studies confirmed that successful spleen transplantation in rodents can result in donor- specific tolerance which eliminates the need for lifetime immunosuppression therapy burdened by severe side effects. However, in some rodents strains combinations allogeneic spleen transplantation intrinsically initiated tolerance but in others combinations caused rejection or even graft- versus- host disease [3]. Various hypotheses have been proposed to solve this phenomenon, but no satisfactory explanation has emerged yet. Thus, we proposed to resolve this dilemma by a new technique of spleen transplantation and peripheral specific donor chimerism detection and evaluation.
The History of Spleen Transplantation
In February 1908 Alexis Carrel performed the first successful spleen replantation on a large yellow dog [4]. In 1952 Simonsen for the first time underlined the role of transplanted spleen as a place of recipient’s individual antibodies production [5, 6]. This assumption was confirmed by Wheeler and colleagues in 1960 on 29 dogs’ homotransplantations without immunosuppression which resulted in severe graft rejection [7, 8]. Meanwhile Bilingham and Brent conducted their laboratory experiments on animals which started the discussion about spleen as immunologically competent organ [9]. Most observations on grafted spleens referred to transplantations proceeded in dogs, that’s why the significant revival occurred after description of heterotopic spleen transplantation in rats by Lee, Orloff and by Coburn in 1969 [10, 11]. This scientific inversion in spleen animal model examination originated a new era of “spleenology”. Exploiting newly developed techniques of spleen allotransplantation, Bitter- Suermann characterized fully MHC mismatched spleen allografts survival [12, 13]. Moreover, different rat strains combinations without any immunosuppressive protocol acquired spleen graft acceptance [14] or lethal graft versus host disease [15]. Another question was how the spleen influenced simultaneously transplanted organs from the same strain. The survival results were unforeseeable in skin, kidney, pancreas, heart and small bowel transplantation. On the one hand spleen allograft induced tolerance over 100 days [16–20] but on the other hand different strains configurations caused short time of rejection [12, 21–25]. The advent of new immunosuppressive and immunomodulation protocols in 1980s turned out to be a good opportunity to check the influence of spleen allotransplantation on organ survival under short non-myeloablative treatment [20, 25, 26]. The results were promising achieving indefinite grafts’ survival. Meanwhile, in other studies, splenocytes had been used for establishing donor specific tolerance as a better clinical application in opposite to spleen transplantation. However, results were ambiguous [27–30]. Nowadays this idea has been revived with success by different types of splenic cells injections [30–33]. Despite the role of spleen allograft transplantation in prolonging the survival of organs which had been well documented, there were also reports showing a lack of correlation between spleen grafting and tolerance. Furthermore, some notifications demonstrated that spleen provoked immunosuppression had no effect on a rate of organ survival [34] and even showed better prolongation of graft survival after splenectomy [35–37].
The Spleen Anatomy, Histology and Function
The spleen is an intraperitoneal organ found in all vertebrate animals and in an adult 250 mg weight rat is approximately 3 ± 1 cm in length. The spleen usually weights 0.48 ± 0.05 (g). Spleen is located vertically on the left side of the cranial abdomen. It lies behind the stomach, high up on the left side of the abdomen. It is secured to the greater curvature of the stomach by the gastrosplenic ligament. It is enclosed in an elastic capsule of connective tissue, which spreads into the parenchyma and divides the organ into lobules. A fine mesh of reticular fibers support the parenchyma forming trabeculae and is divided into two types of tissue, separated by the marginal sinus, called red and the white pulp. The white pulp is lymphatic tissue consisting mainly of lymphocytes around arteries. The red pulp comprise of venous sinuses with blood and cords of lymphatic cells [38].
Vascularization
The splenic artery is the largest branch of the celiac trunk which follows a tortuous course. The splenic vein collects branches from the stomach, pancreas and inferior mesenteric vein. Splenic vein joins the superior mesenteric vein and the hepatic portal vein. The splenic artery and vein enter the spleen through the hilum and divide into six or more branches (brush arteries). The splenic artery is slightly superior to the vein [39]. The artery branches divide into arterioles and capillaries, which may either connect with the venous sinuses, or terminate with open ends in the splenic cords. Blood is released into the splenic cords, either from the sinuses and capillaries, eventually filters back into the sinus network [40].
The sinuses converge and empty into trabecular veins, which then merge into a single splenic vein [41].
The spleen is a major lymphoid and blood filtration organ. It is responsible for storing and removing erythrocytes from the blood as well as antigen surveillance of the blood and antibody production (secondary lymphoid organ). In the fetus (during haematopoietic transitional phase) the spleen also plays a role in hematopoiesis, it becomes the main erythrocyte producing organ [42, 43].
Different Methods of Spleen Transplantation
Anesthesia Protocol
Operations were performed under anesthesia cocktail of ketamine (30 mg/kg), xylazine (6 mg/kg) and acepromazine (1 mg/kg). Supplementary doses were given if necessary. Postoperative pain was defeated by postoperative pain systemic analgesic techniques (combined: non-steroidal anti-inflammatory drugs, paracetamol and opioids).
There are several steps of the transplantation process. The surgical procedure of spleen allograft transplantation was accomplished in two stages: harvesting the graft and recipient place preparation with anastomosis the donor’s vessels with the recipients’.
Harvesting the Spleen
Each spleen transplantation begins with the organ harvesting stage. After anesthesia has taken effect before the surgery, the abdomen regions are trimmed and swabbed with antiseptic solution. The donor rat is fixed in supine position. Using middle incision moving intestines to the right and stomach to the diaphragm, the splenic pedicle is mobilized by isolating portal and splenic vessels. The ligaments between spleen, pancreas, stomach and intestines are dissected. This advance warrant the proper ligation of the celiac axis preserving the splenic artery and releasing the aorta. The vascular branches running from and to the stomach are ligated and divided, as well as the branches running to the mesenteric artery. In Cleveland Clinic Laboratory to reduce time of consumption ligation titanium clamps are used. Then the aorta is ligated 2–5 mm below celiac axis. The rest of this procedure is performed after preparation of the recipient site for grafting. The spleen is perfused in vivo with Heparin in Ringer’s (50 ml) or Hartmann’s (2 ml) solution using 0.5 ml syringe with 31 G needle. Prior to portal vein ligation 2–5 mm behind confluence of superior mesenteric vein all the collects branches from the stomach, pancreas and the large intestine are ligated. Sometimes in order to obtain better dissection duodenum and gastroduodenal vein are divided. After aorta and portal vein double successive clamping, portal venous and aortic segments are excised “en block” as arterial and venous cuffs [10, 11, 14, 44–46]. In Cleveland Clinic Laboratory for better vessels complementary, instead of using aorta segment or cuff technique (discrepancy in diameter) the celiac trunk after all branches ligation is utilized.
Recipient Place Preparation and Transplantation
The spleen transplantation technique can be divided into orthotopic and heterothopic procedure.
Orthotopic Spleen Allotransplantation Procedure
Small diameters of spleen artery and vein stumps cause that the orthotopic spleen transplantation is a very technical demanding procedure with high risk of anastomotic and thrombotic complications. This is the reason why this method doesn’t obtain the researchers’ approval. Moreover, this procedure involves total resection of recipient’s spleen with all side effects. In fact the preparation procedure of graft implantation is similar to the donor spleen harvesting technique until the moment of portal and splenic vessels’ branches isolation and ligation. The splenic artery is dissected just 5 mm from the aorta. The splenic vein is ligated and transected at least 5 mm before the place of confluence with mesenteric superior vein. The ligation of mesenteric inferior vein is demanded. The vessels stumps are being prepared to anastomosis end- to- end with the donor vascular spleen pedicle.
Heterotopic Spleen Allotransplantation Procedure
In 1969 Lee, Orloff and separately Coburn published the rat model of heterotopic spleen transplantation [10, 11]. They described method of utilizing donor’s aortic and portal vein segments for end-to-side anastomoses with the aorta and vena cava of the recipient’s spleen. The implantation of the allograft presented by Müller and Wunderlich [47] differ from other by utilize the renal artery and vein post nephrectomy. The splenic pedicle (celiac artery and portal vein) was anastomosed end-to end with the stumps of renal artery and vein. This authors assumed that this end-to-end method avoids cross-clamping of the aorta and vena cava.
To cope with the size of the recipient’s renal artery they used the celiac artery itself, an artery on small aortic patch or an aortic segment for lumen compatibility. The main disadvantages of this methods were: invasive abdomen surgery and involvement of large vessels in transplantation procedure.
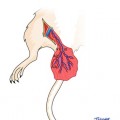
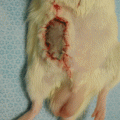
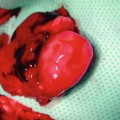
In our laboratory we invented a new less harmful for recipient method of spleen allograft transplantation (Fig. 48.1). In the recipient rat, following incision of the right groin skin the right femoral vessels were exposed, and dissected proximally up to the inguinal ligament and distally down to the branching from the superficial epigastric vessels, ligated and cut there. The donor’s spleen pedicle was anastomosed with the recipient’s femoral artery and vein using the standard end-to-end microsurgical procedure with 10.0 nonabsorbable nylon (Ethicon) and interrupted or continuous suture technique (Fig. 48.2). Moreover this method of spleen transplantation create an easy biopsies access for histopathological evaluation. The rejection can be easy noticed and be less hazard for the recipient (Fig. 48.3).
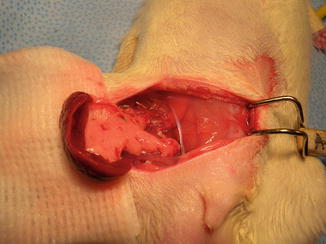
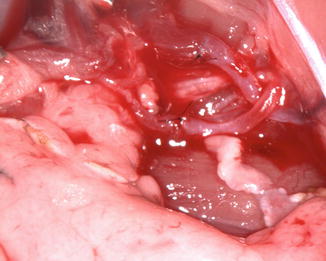
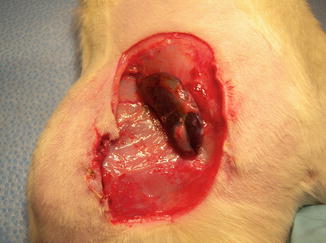

Fig. 48.1
The donor ACI (RT1a) spleen after into Lewis (RT11) groin area allotransplantation

Fig. 48.2
The donor ACI (RT1a) spleen and recipient Lewis (RT11) vessels just after anastomosis

Fig. 48.3
A donor ACI (RT1a) spleen after 100 days post transplantation. The spleen was in a good condition without any rejection symptoms
The Immunologic Potential of the Spleen in Tolerance Induction
The immunological tolerance would eliminate the need of chronic immunosuppressive drug therapy. Spleen is a single secondary lymphoid organ with reticuloendothelial system which plays a major role in both adaptive and innate immune response [2]. The tolerogenic capability of spleen has been confirmed in several rodent models of spleen allotransplantations. For successful donor’s spleen transplantation in rodents is responsible donor’s skin and solid organ tolerance induction even without immunosuppressive protocols [48]. Studies investigating the immunologic aspects of spleen tolerance induction showed different approaches to explain this phenomenon. To better clarify this complex issue we can divide it into particular issues:
1.
Spleen split tolerance.
2.
Splenic chimerism induction.
Spleen Split Tolerance
Secondary lymphoid organs (the spleen, lymph nodes and mucosal lymphoid tissues) provide the proper environment for antigen-presenting cells to interact with activate naive T and B lymphocytes [49]. The prevailing studies suggest that spleen’s role in graft rejection is different in vascularized and non-vascularized organ transplantation model [20, 50–53]. One possible explanation is that spleen comprise two opposite functions: inducing graft rejection and graft prolongation [54]. Moreover, the results suggest that the spleen allotransplantation creates a balance between host-versus-graft and GVH responses, leading to long-term survival of both the spleen and co-transplanted donor’s organ [3].
Better understanding of the specifics of donor vs recipient DCs (Dendritic cell) [55] and trafficking anti-allograft immune responses into T cell areas of secondary lymphoid tissues [56] with profound exploration of APCs (Antigen Presenting Cell) migration routes [57] create a convincing background for filter migration hypothesis [54]. In non-vascularized skin allograft model donor’s and recipient’s APCs migrate to the spleen through regional lymph node.
The lymph node plays a filter role and traps activated regulatory or immature DS which generate effector T cells. Passing through the lymph node which is a selection zone, APCs engraft the spleen and generate Treg. The APCs cells can induce proliferation of alloantigen- specific CD4+/CD25+ regulatory T cells in a recipient’s spleen [58]. The splenectomized rat model confirms this hypothesis [59].
In vascularized graft the DCs migrate directly through the blood to spleen and activate the rejection cascade [60]. Nevertheless the spleen generates and maintains tolerogenic CD8+ Tregs confirmed by rejection of second donor graft upon splenectomy long-term after transplantation [61]. Moreover spleen is responsible for maintaining CD4+/CD25+ regulatory T cells. To contradict sensitizing of alloantigen specific effector cells which is an unfavorable situation, the immunosuppression protocol attenuating the recipient immune system is demanded. This theory is complementary with Siemionow and coauthors findings about vascular and nonvascular mechanism of acceptance [62].
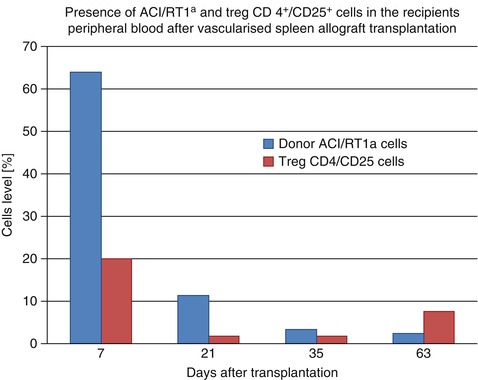
In Cleveland Clinic Laboratory we performed seven spleen allotransplantation between ACI (RT1a) donors and Lewis (RT11) recipients, under 7 days protocol of αβ-TCRmAb/CsA therapy. We obtained over 100 days after transplantation graft survival. Our observations indicated the multilineage donor specific (RT1a) peripheral blood chimerism on day 63 which achieved 2,44 % however the level of regulatory T cells resulted 7,66 % (Fig. 48.4). The preliminary results are very promising and underlining the role of regulatory T CD4+/CD25+ cells in a tolerance induction by allogenic spleen transplantation.
Get Clinical Tree app for offline access

Fig. 48.4
Comparison the level of donor ACI/RT1a




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree