Fig. 52.1
Spitzoid melanoma. An erythematous fast-growing nodule on the upper arm of a 49-year-old man
Pathology
Spitzoid melanoma shares many histopathologic features with Spitz nevus, and it is one of the most difficult and problematic diagnoses in dermatopathology. The mainstay for the diagnosis is nests of variable size and shape with large spindle or epithelioid cells characterized by abundant eosinophilic cytoplasm and prominent eosinophilic nucleoli. Although there are no specific histopathologic diagnostic criteria for spitzoid melanomas, these malignancies are usually asymmetrical, lack both circumscription and maturation, and exhibit high cell density, deep mitoses, an expansive growth pattern and consumption of the epidermis or ulceration (Figs. 52.2–52.6).
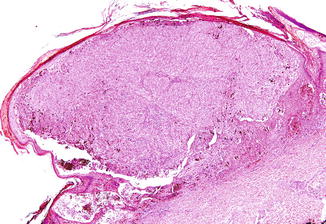

Fig. 52.2
Spitzoid melanoma. An asymmetrical, expansive nodule showing high cell density and consumption of the epidermis
Compared to Spitz nevus, the melanocytic epidermal component is usually not well demarcated with spreading of melanocytes at the edges. The junctional nests are irregularly shaped and unevenly distributed with irregular pseudoacantholytic clefts, and in some areas, single melanocytes predominate showing a pagetoid spreading (Fig. 52.3). The dermal component is characterized by an asymmetrical nodular proliferation of irregular and crowded nests with enlarged, atypical epithelioid or spindle-shaped melanocytes that are often amelanotic and lack maturation (Figs. 52.4 and 52.5). The phenomenon of zonation (similar appearance of melanocytes along horizontal levels of the lesion) is usually absent. Spitzoid melanoma has a higher degree of cytologic atypia with more pleomorphic, hyperchromatic nuclei that contain large or multiple nucleoli (Figs. 52.7 and 52.8). The deep dermal component shows an expansile growth that involves the superficial subcutaneous tissue rather than an infiltrative pattern. Melanocytes in mitosis are an important clue; more than three dermal mitoses per high-power field, mitoses in the deeper parts of the lesion, and atypical mitoses favor a diagnosis of spitzoid melanoma. The epidermis may ulcerate or is atrophic showing epidermal consumption. Kamino bodies are not features of spitzoid melanoma, and when they are present in a spitzoid neoplasm, a diagnosis of nevus is favored. A dense inflammatory lymphoplasmacytic infiltrate, lack of adnexal structures, areas of necrosis, solar elastosis, regression with or without melanosis, vascular invasion, and neural infiltration all are features that favor melanoma. Recently, five subtypes of spitzoid melanoma have been suggested, i.e., “genuine” similar to small compound Spitz nevus, “uniform” including intradermal spitzoid neoplasm composed of a sheet of epithelioid cells with radical absence of adnexal appendages and little or any collagen, “packed” including intradermal spitzoid neoplasm with very compact nests and dermal artifactual breakages, “polypoid,” and “pigmented” including compound spitzoid neoplasm with striking amounts of melanin both in superficial and deep dermis with irregular distribution and melanophages.
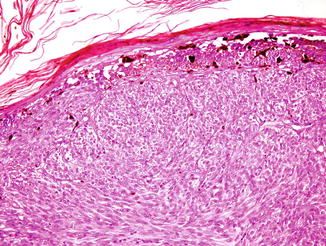
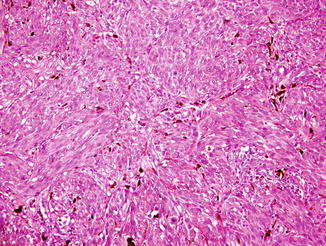
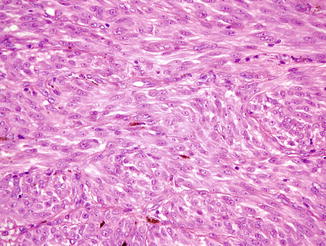

Fig. 52.3
Spitzoid melanoma. A spindle-shaped and epithelioid eosinophilic proliferation. The junctional nests are irregularly shaped and unevenly distributed with irregular pseudoacantholytic clefts

Fig. 52.4
Spitzoid melanoma. The dermal component is characterized by a nodular proliferation of irregular and crowded nests with enlarged, atypical epithelioid, or spindle-shaped melanocytes lacking maturation

Fig. 52.5
Spitzoid melanoma. Atypical epithelioid or spindle-shaped melanocytes with abundant eosinophilic cytoplasm and prominent eosinophilic nucleoli. Deep mitoses are present in the deep dermal component
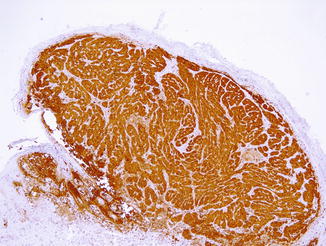
While no specific immunohistochemical marker or molecular and genetic test still exists to distinguish Spitz or spitzoid benign neoplasm from spitzoid melanoma, a combination of immunohistochemical stains has been proposed as a useful tool. This panel includes HMB45, (Fig. 52.9), Ki-67/MIB-1, CD99, p16, Neuropilin-2, Bcl-2, cyclin D1, p53, and p21 among others (Figs. 52.6, 52.7, 52.8 and 52.9). HMB45 is evaluated according to a maturation gradient. If the immunostain is expressed throughout all of the lesion or in a patchy distribution to include deep melanocytic nests, this is considered a sign of malignancy. If there is positive staining at the top of the lesion with loss of staining in the deeper part into the dermis, this favors Spitz nevus. Ki-67/MIB-1 is suggested to be a useful marker in thick and noninflammatory neoplasms and a nuclear proliferation index of 10 % favors a diagnosis of melanoma. CD99 is reported to be expressed in 56 % of spitzoid melanomas in a strong and diffuse pattern but only in 5 % of Spitz nevi. Loss of both cytoplasmic and nuclear expression for p16 is present in spitzoid melanomas as compared with Spitz nevus without any correlation with its Breslow thickness. However, the value of p16 expression has been recently questioned as loss of p16 staining does not necessarily reflect malignancy, at least in spitzoid melanoma of adulthood type. Neuropilin-2 is a cytoplasmic/cell surface protein that is a mediator of melanoma-endothelial cell interaction and has been found to be expressed by spitzoid melanoma, while most Spitz nevi are negative. A wider number of cases and a long-term follow-up are needed to determine whether immunohistochemistry has any predictive value in the evaluation of spitzoid lesions.