Abstract
Human survival depends on the ability to function effectively within a dry external environment. The epidermis functions as a barrier that maintains body hydration and excludes harmful external agents. The skin barrier is acquired via the process of epidermal terminal differentiation during which keratinocytes transform into corneocytes. The latter are characterized by a cornified envelope that encases densely packed keratin filaments cemented by several proteins, in particular filaggrin. The corneocytes have been likened to “bricks” and are surrounded by a lipid-rich extracellular matrix, analogous to “mortar”. This matrix is organized as lamellar membranes that are derived from precursor lipids secreted from lamellar bodies and it mediates the permeability barrier in the outermost stratum corneum. The key lipids are cholesterol, free fatty acids and ceramides, with de novo lipid synthesis within the epidermis playing a major role in their production. Disruption of these biochemical pathways can lead to an impaired skin barrier. Disorders with an impaired skin barrier are intimately linked to cutaneous inflammation (outside-in barrier) and conversely inflammation can impair the barrier (inside-out barrier). An increasing number of both common and rare skin diseases have been found to be driven by an impaired skin barrier.
While the skin barrier performs important protective physiologic functions, it also impedes the delivery of drugs into the skin. Percutaneous drug absorption is influenced by both drug concentration and the drug carrier vehicle. In addition, chemical and physical enhancers can be used to enhance penetration of topical formulations and transdermal patches. Percutaneous drugs produce effects that are local (e.g. corticosteroids) as well as systemic (e.g. nicotine, scopolamine).
Keywords
skin barrier, impaired skin barrier, lamellar bodies, stratum corneum, cholesterol, cholesterol sulfate, ceramides, phospholipids, corneocytes, keratinocytes, epidermal terminal differentiation, permeability barrier, chemical enhancer, drug vehicles, epidermal lipid metabolism, skin permeability, transdermal drug delivery, topical formulations, percutaneous drug absorption, transdermal patches, transepidermal absorption
Skin Barrier
- Matthias Schmuth
- Peter M. Elias
- Thomas J. Franz
- Jui-Chen Tsai
- Gopinathan K. Menon
- Kenneth R. Feingold
- Peter M. Elias
Chapter Contents
Stratum corneum structure and organization 2176
Epidermal metabolism and the skin barrier 2176
Impaired skin barrier and cutaneous inflammation 2179
The skin provides the largest interface between the human body and the external environment. Therefore, one of its most important functions is to prevent ingress of potentially harmful exogenous substances (outside-in barrier). Likewise, the skin must prevent excessive loss of water and other bodily constituents given that desiccation is incompatible with life (inside-out barrier).
The skin’s remarkable barrier properties are due in large part to epidermal homeostasis, which depends on several cell types and function . As they differentiate, keratinocytes accumulate specialized organelles, e.g. lipid-containing lamellar bodies, whose contents play a key role in the formation of the stratum corneum ( Fig. 124.1 ). The corneocytes within the stratum corneum are composed primarily of aggregated keratin filaments encased in a cornified envelope and have been likened to “bricks”. They are surrounded by an extracellular milieu of lipids organized as multiple lamellar bilayers, analogous to “mortar”. These structured lipids prevent excessive loss of water from the body and likewise block entry of exogenous compounds, including many topically applied drugs (except for those that are lipid-soluble and of low molecular weight).
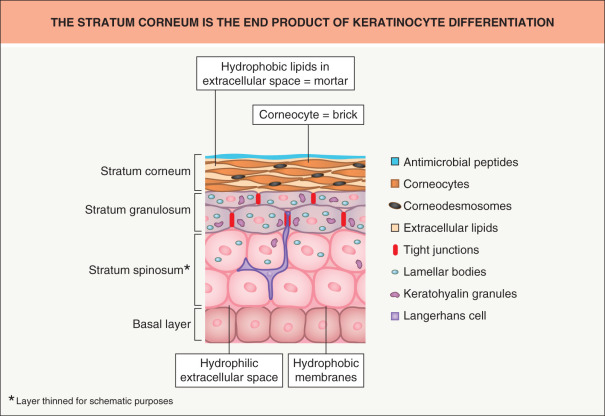
Originally, the skin barrier was thought to be formed solely by lipid secretion and processing . However, more recently it was shown that tight junctions not only play an important role in directly blocking passage of some molecules, but also direct epidermal differentiation and formation of the lipid barrier . Of note, the tight junction barrier develops before the lipid barrier, both in a fetal rat model and in human epidermal equivalents (HEE), and tight junction expression recedes as the lipid barrier develops .
Stratum Corneum Structure and Organization
The stratum corneum is a composite material made of proteins and lipids structurally organized as “bricks and mortar” (see Fig. 124.1 ; Table 124.1 ) . Instead of being uniformly dispersed, the highly hydrophobic lipids in normal stratum corneum are sequestered within the extracellular spaces, where this lipid-enriched matrix is organized into lamellar membranes that surround the corneocytes . Hence, rather than stratum corneum thickness, variations in number of lamellar membranes (= lipid weight %), membrane structure, and/or lipid composition provide the structural and biochemical basis for site-related variations in permeability . It follows, then, that the extracellular, lipid-enriched matrix of the stratum corneum comprises not only the structure that limits transdermal delivery of hydrophilic drugs, but also the so-called stratum corneum “reservoir” , within which lipid-soluble drugs (e.g. topical corticosteroids) can accumulate and be slowly released.
| FEATURES OF THE STRATUM CORNEUM |
|
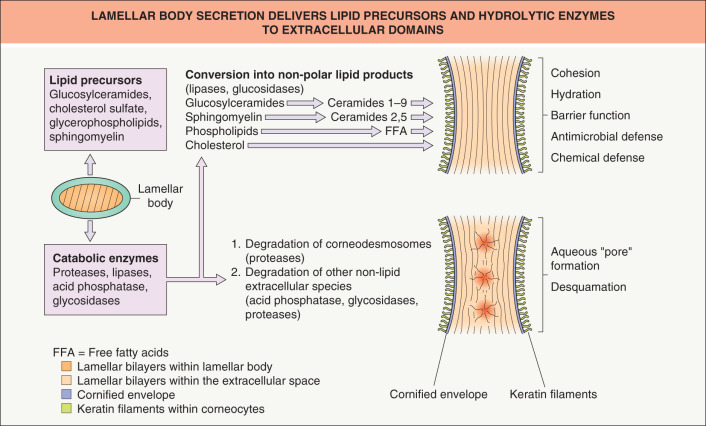
Human stratum corneum is typically comprised of about 20 corneocyte cell layers, which differ in their thickness, packing of keratin filaments, filaggrin content and number of corneodesmosomes, depending on body site. Corneocytes are surrounded by a highly cross-linked, resilient sheath, the cornified envelope, while the cell interior is packed with keratin filaments embedded in a matrix composed mainly of filaggrin and its breakdown products (the latter are also referred to as “natural moisturizing factors”). As noted previously, individual corneocytes, in turn, are surrounded by a lipid-enriched extracellular matrix, organized largely into lamellar membranes, which are derived from secreted lamellar body precursor lipids ( Fig. 124.2 ). Following secretion, lamellar body contents fuse end-to-end, forming progressively elongated membrane sheets , a sequence requiring the action of a battery of lipolytic “processing” enzymes (see below).
The exceptionally low permeability of normal stratum corneum to water-soluble drugs is the consequence of several characteristics of the lipid-enriched, extracellular matrix ( Table 124.2 ) . Moreover, not only the paired-bilayer arrangement of extracellular lipids, but also their extreme hydrophobicity and the composition and distribution of the three key species (ceramides, cholesterol and free fatty acids) in a critical (1 : 1 : 1) molar ratio, are further characteristics that provide for barrier function.
| FACTORS AFFECTING HOW STRATUM CORNEUM LIPIDS MEDIATE BARRIER FUNCTION |
|
Ceramides account for ~50% of the total stratum corneum lipid mass and are crucial for the lamellar organization of the stratum corneum barrier . Of the nine ceramide classes, acylceramides or ceramides 1, 4 and 7 (which contain ω-hydroxy-linked essential fatty acids in an ester linkage) are epidermis-unique compounds, known to be important for barrier function . Cholesterol , the second most abundant lipid by weight in the stratum corneum, promotes the intermixing of different lipid species and regulates its “phase” behavior . Free fatty acids , which account for 10–15% of stratum corneum lipids, consist predominantly of very-long-chain, saturated species with ≥18 carbon atoms. A decrease in the concentrations of any of these critical lipid species compromises barrier integrity due to alteration of the molar ratio of the membranes that mediate normal barrier function.
Epidermal Metabolism and the Skin Barrier
Biosynthetic Activities
Epidermal differentiation is a vectorial process that is accompanied by dramatic changes in lipid composition, including loss of phospholipids with the concomitant emergence of ceramides, cholesterol and free fatty acids in the stratum corneum (see Fig. 124.2 ). Although epidermal lipid synthesis is both highly active and largely autonomous from systemic influences, it can be regulated by external influences, i.e. changes in the status of the permeability barrier . Acute perturbations of the permeability barrier stimulate a characteristic recovery sequence that leads to restoration of normal function over about 72 hours in young skin (the cutaneous stress test). This sequence includes an increase in cholesterol, free fatty acid and ceramide synthesis that is restricted to the underlying epidermis and is attributable to a prior increase in mRNA and enzyme activity/mass for each of the key synthetic enzymes ( Fig. 124.3 ). Furthermore, synthesis of each of the three key lipids is required for normal barrier homeostasis in that topically applied inhibitors of the key enzymes in each pathway produce abnormalities in permeability barrier homeostasis .
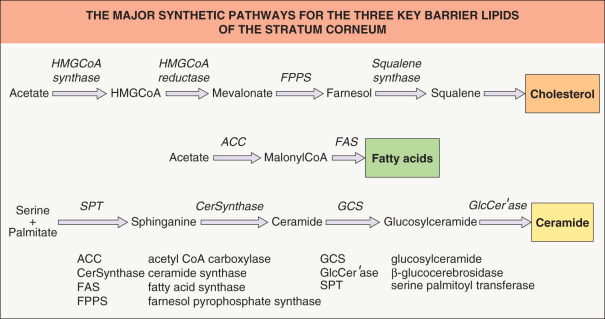
Lamellar Body Secretion
The unique two-compartment organization of the stratum corneum is attributable to the secretion of lamellar body-derived lipids and co-localized hydrolases at the stratum granulosum–stratum corneum interface . Under basal conditions, lamellar body secretion is slow, but sufficient to provide for barrier integrity. Following acute barrier disruption, calcium is lost from the outer epidermis and much of the preformed pool of lamellar bodies in the outermost cells of the stratum granulosum is quickly secreted . Calcium (Ca 2+ ) is an important regulator of lamellar body secretion, with the high levels of Ca 2+ in the stratum granulosum restricting lamellar body secretion to low, maintenance levels .
Extracellular Processing
Extrusion of the polar lipid contents of lamellar bodies at the stratum granulosum–stratum corneum interface is followed by the processing of those lipids into more hydrophobic species that form mature lamellar membranes ( Fig. 124.4 ). The extracellular processing of glucosylceramides, phospholipids and cholesterol sulfate resulting in the accumulation of ceramides, free fatty acids and cholesterol within the stratum corneum is attributable to the co-secretion of a set of hydrolytic enzymes (see Fig. 124.2 ).
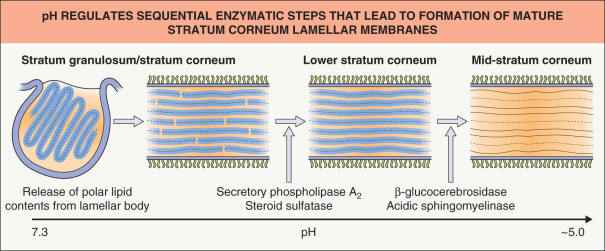
Extracellular processing of glucosylceramides plays a key role in barrier homeostasis (see legend to Fig. 124.3 ). In addition, phospholipid hydrolysis, catalyzed by one or more secretory phospholipases (e.g. sPLA 2 ), generates a family of nonessential free fatty acids, which are also required for barrier homeostasis . Since applications of either bromphenacylbromide or MJ33 (chemically unrelated sPLA 2 inhibitors) modulate barrier function in intact skin, sPLA 2 appears to play a critical role in barrier homeostasis . Moreover, applications of either inhibitor to perturbed skin sites delay barrier recovery.
Sphingomyelin hydrolysis by acidic sphingomyelinase generates two of the nine ceramides required for normal barrier homeostasis (see Fig. 124.2 ). Moreover, patients with mutations in the gene encoding acidic sphingomyelinase that lead to low enzyme activity (Niemann–Pick, Type A) display an ichthyosiform dermatosis, and transgenic mice with an absence of acidic sphingomyelinase also demonstrate a barrier abnormality. Finally, applications of nonspecific inhibitors of acidic sphingomyelinase to perturbed skin sites lead to a delay in barrier recovery .
Just as with glucosylceramides and sphingomyelin, cholesterol sulfate content increases during epidermal differentiation, and then decreases progressively as the latter is desulfated during passage from the inner to the outer stratum corneum . Both cholesterol sulfate and its processing enzyme, steroid sulfatase, are concentrated in membrane domains of the stratum corneum. Of note, the content of cholesterol sulfate in these sites is increased by ~10-fold in recessive X-linked ichthyosis (see Ch. 57 ) . Not only is recessive X-linked ichthyosis characterized by a barrier defect , but repeated applications of cholesterol sulfate to intact skin can produce a barrier abnormality . In both cases, the barrier abnormality is attributable to cholesterol sulfate-induced phase separation within lamellar membrane domains . But the barrier defect may also be, in part, attributed to a reduction in cholesterol content, since cholesterol sulfate is a potent inhibitor of HMG-CoA reductase (see Fig. 124.3 ).
In addition to lipid precursors and hydrolytic enzymes (e.g. steroid sulfatase, acidic sphingomyelinase), lamellar bodies contain proteases and antiproteases that orchestrate the orderly digestion of corneodesmosomes, allowing corneocyte shedding. These organelles also release antimicrobial peptides, including human β-defensin 2 and LL-37 (the carboxy-terminal fragment of human cathelicidin).
Acidification
The fact that the stratum corneum displays an acidic external pH (“acid mantle”) is well documented, but its origin is not fully understood. Acidification of the extracellular space could be explained by: (1) extraepidermal mechanisms, including surface deposits of eccrine gland- and sebaceous gland-derived products as well as metabolites of microbial metabolism; (2) endogenous catabolic processes, e.g. phospholipid-to-free fatty acid hydrolysis, deamination of histidine to urocanic acid; and/or (3) local generation of protons within the lower stratum corneum by sodium-proton antiporters [NHE 1 ] inserted into the plasma membrane . These mechanisms would explain not only the pH gradient across the interstices of the stratum corneum (see Fig. 124.4 ), but also selective acidification of membrane microdomains within the lower stratum corneum.
The concept that acidification is required for permeability barrier homeostasis is supported by the observation that barrier recovery is delayed when acutely perturbed skin sites are immersed in neutral pH buffers or when either the sodium–proton exchanger/antiporter or sPLA 2 -mediated phospholipid catabolism to free fatty acids is blocked . Acidification appears to impact barrier homeostasis through regulation of enzymes involved in extracellular processing, such as β-glucocerebrosidase and acidic sphingomyelinase, which exhibit acidic pH optima (see Fig. 124.4 ).
Impaired Skin Barrier and Cutaneous Inflammation
Abnormalities of the skin barrier are observed in both common and rare skin diseases. Many disorders of cornification, including the ichthyoses, are due to mutations in genes that encode key components of the skin barrier such as corneocytes and extracellular lipids . In atopic dermatitis, mutations in the gene encoding filaggrin commonly drive inflammation (see Ch. 12 ) , and psoriasis susceptibility genes include those for cornified envelope proteins LCE3B and LCE3C . Fig. 124.5 depicts the impact of reduced filaggrin production on various functions of the stratum corneum.
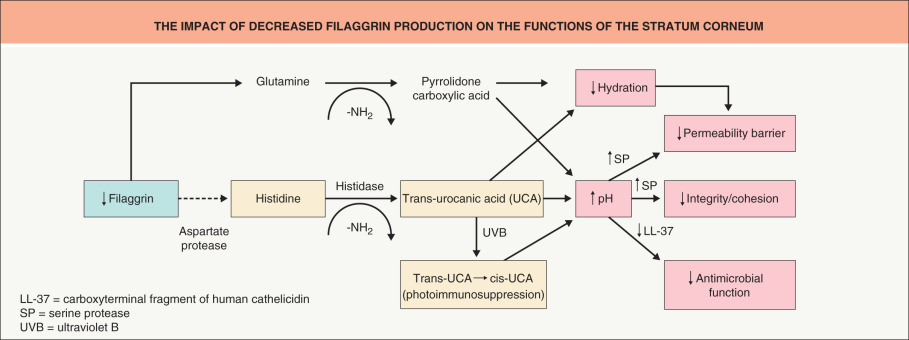
Not only can barrier disruption lead to inflammation, but inflammation can impair skin barrier function , thus linking the immune system and the permeability barrier. In atopic dermatitis, inflammation reduces expression of tight junctions and antimicrobial peptides, with the latter explaining in part the predisposition to colonization with Staphylococcus aureus . In addition, abnormalities in serine protease/antiprotease expression are observed in some patients with ichthyosis (e.g. Netherton syndrome) as well as atopic dermatitis. Our increasing knowledge about the pathophysiology of skin barrier-driven diseases offers major opportunities for novel therapies of both monogenic and polygenic skin diseases .
Transdermal Drug Delivery
- Mark R. Prausnitz
- Peter M. Elias
- Thomas J. Franz
- Jui-Chen Tsai
- Gopinathan K. Menon
- Kenneth R. Feingold
- Peter M. Elias
Chapter Contents
Parameters affecting skin permeability 2180
Strategies to enhance transdermal drug delivery 2182
Parameters Affecting Skin Permeability
As discussed in greater detail in the following sections, the skin is an attractive site for drug delivery . However, normal skin provides a significant barrier to drug absorption. Understanding the parameters that affect the permeability of this barrier is essential for achieving successful drug therapy via the skin. While local cutaneous effects are generally achieved by dissolving or suspending the drug in a vehicle that is applied topically as a semi-solid formulation (e.g. cream or ointment) , administration of systemic therapy via the skin is typically accomplished through the use of a patch. In either situation, drug is supplied at the surface of the skin for diffusion across the stratum corneum, with the goal of reaching therapeutic targets within the skin and/or systemic uptake via superficial dermal capillaries.
Parameters Controlling Absorption
Conventional transdermal drug delivery is a passive process governed by Fick’s law, that is, the rate of absorption or flux ( J ) of any substance across a barrier is proportional to its concentration difference across that barrier . For topically applied drugs , the concentration difference can be simplified as the concentration of drug in the vehicle, C v , and the proportionality constant relating flux to concentration is the permeability coefficient, K p ( Eq. 1 ). K p is composed of factors that relate to both drug and barrier, as well as their interaction. These factors are: D , the diffusion coefficient; K m , the partition coefficient; and L , the length of the diffusion pathway ( Eq. 2 ). Thus, four factors control the kinetics of percutaneous drug absorption ( Eq. 2 ); however, it is of great practical importance that two of the four ( C v , K m ) are highly dependent on one additional factor, the vehicle.
J = K p C v
J = ( D K m L ) C v
Role of the Vehicle
The vehicle is an important link between drug potency and therapeutic effectiveness, since extensive pharmaceutical research has shown that the composition of the vehicle can profoundly influence the rate and extent of absorption (bioavailability). As illustrated by the potency ranking scale for glucocorticoids , the same drug appears in different potency classes when formulated in different vehicles ( Table 124.3 ). It was once axiomatic that ointments were more potent than creams. Though true for the early glucocorticoid products, it is no longer generally applicable. Greater understanding of the science underlying topical formulations has allowed creams, gels, solutions, and foams to be specifically formulated equipotent to ointments (see Table 124.3 ).
| EFFECT OF VEHICLE ON POTENCY | |
|---|---|
| Corticosteroid * | Potency class |
| Betamethasone dipropionate | |
| 1 |
| 1 |
| 2 |
| 2 |
| 3 |
| 5 |
| Clobetasol propionate | |
| 1 |
| 1 |
| 1 |
| 1 |
| 1 |
| 2 |
| Fluocinonide | |
| 2 |
| 2 |
| 2 |
| 2 |
| 3 |
| Triamcinolone acetonide | |
| 3 |
| 4 |
| 5 |
| 6 |
In the rational design of dermatologic vehicles that maximize bioavailability, two factors are of critical importance: (1) solubilizing the drug in the vehicle ( C v ); and (2) maximizing movement (partitioning) of drug from vehicle to stratum corneum ( K m ). The partition coefficient describes the ability of a drug to escape from the vehicle and move into the outermost layer of the stratum corneum. It is defined as the equilibrium solubility of drug in the stratum corneum (sc) relative to its solubility in the vehicle ( K m = C sc / C v ).
Drug Concentration
The driving force for percutaneous absorption is the concentration of soluble drug in the vehicle. Many older topical drug products were marketed with the expectation that higher concentrations were more potent. Although true for some products such as tretinoin gels and creams (0.01–0.1%), in which the drug is completely solubilized at all concentrations, for others it is not the case. Hydrocortisone 1% and 2.5% in a cream formulation have been shown to be of equal potency, as have triamcinolone acetonide 0.025%, 0.1% and 0.5% creams . One of the major advances in formulating glucocorticoids, as first shown with fluocinonide, came when it was discovered that the addition of propylene glycol to the vehicle could completely solubilize the drug. This led to corticosteroid products with greater potency, as demonstrated in the vasoconstrictor assay.
Newer products are now tested during the development process to ensure that increased drug concentration results in increased bioavailability. However, excess non-dissolved drug can sometimes be advantageous, especially in transdermal patches worn for prolonged periods of time (e.g. up to a week). In this situation, as dissolved drug is absorbed into the body, non-dissolved drug can then become dissolved in order to maintain an equilibrium, thereby maintaining a constant dissolved drug concentration over time and providing a constant rate of delivery .
Partition Coefficient
In general, topically applied drugs are poorly absorbed because only a small fraction partitions into the stratum corneum. Most of the drug remains on the skin surface, subject to loss from a multitude of factors (exfoliation, sweating, wash-off, rub-off, adsorption onto clothing, and chemical or photochemical degradation). Even 10–12 hours following application, a drug that has not been lost by exfoliation or rub-off remains largely on the skin surface, and it is easily removed by a simple soap and water wash . In the case of patches worn for several days, as much as half of the original amount of drug may still be present in the patch when it is removed, and this can pose a safety hazard upon disposal, especially with potentially dangerous drugs such as fentanyl .
A number of physical and chemical factors can improve partitioning. Hydration of the skin due to occlusion, either from a topical formulation or a patch, expands the reservoir volume available to drugs within the stratum corneum; this can increase absorption by as much as five- to ten-fold . Common excipients such as ethanol and propylene glycol can also alter barrier structure so as to increase partitioning . In addition, many excipients have good solvent properties and, as a result, positively affect C v as well as K m . The use of high concentrations of propylene glycol to maximize bioavailability has become pervasive among the super- and high-potency corticosteroids, but at a price. Adverse events such as burning and stinging are common when such preparations are applied to fissured or eroded skin, and contact dermatitis may occur.
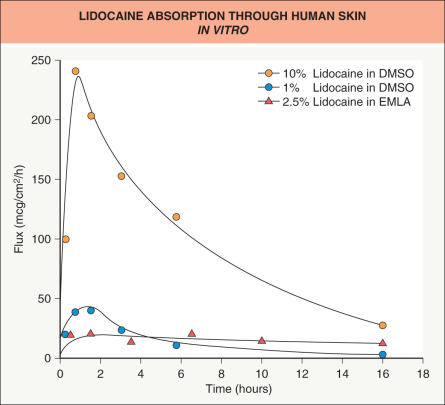
A number of other compounds have been identified as enhancers. Dimethylsulfoxide (DMSO), the archetypical enhancer, exemplifies the effects that can be achieved ( Fig. 124.6 ). As with ethanol and propylene glycol, both C v and K m are affected. Because DMSO is a superb solvent, higher drug concentrations can be achieved than with other solvents. In addition, DMSO expands the stratum corneum barrier, permitting increased drug uptake and possibly an increased rate of diffusion ( D ) through the barrier. However, the use of powerful enhancers such as DMSO is constrained by excessive skin irritation or toxicity .
Regional Variation
All body sites are not equally permeable . Variations in stratum corneum thickness and lipid composition, the number of sebaceous glands, and hydration status can all affect absorption. Current data and clinical experience suggest that one can crudely rank regional permeability as follows: nail < < palm/sole < trunk/extremities < face/scalp < < scrotum.
Strategies to Enhance Transdermal Drug Delivery
Despite the significant permeability barrier of the stratum corneum, drug delivery via the skin is a very attractive option and is widely employed for both local and systemic therapy ( Table 124.4 ; Fig. 124.7 ) . Topical treatment of cutaneous disorders obviously targets the site of disease, thereby minimizing adverse side effects elsewhere within the body. Delivery of systemic therapies via the skin avoids degradation of the medication within the gastrointestinal tract and from first-pass metabolism by the liver, both of which are associated with oral administration of drugs, in addition to evading the pain and safety issues associated with injections. Transdermal delivery of drugs, especially via long-acting patches, enables infrequent dosing and maintenance of steady-state drug levels.