(1)
Department of General and Vascular Surgery, Poznan University of Medical Sciences, Poznan, Poland
Abstract
Extensive trauma can lead to the peripheral nerve transection injury. Further reconstruction can be done with the use of the best method: end-to-end coaptation, if this will not cause tension of the approximated trunks. Larger nerve gaps resulted from tissue loss or degeneration, require additional material for the reconstruction. Ideal material acceptable for nerve engraftment is autologous nerve, however the sources of it are limited. Despite well-established method of full-thickness cable-nerve grafting there is still need for research to improve results of functional nerve restoration following grafting, as well as to increase the amount of neural tissue material suitable for reconstruction.
An experimental rat animal model of the single fascicle graft repair of the sciatic nerve gap, had been developed to evaluate the idea of large nerve defect reconstruction with the lowest possible amount of autologous neural material needed for effective nerve regeneration. In this chapter the scientific rational for the use of single fascicle graft nerve repair, description of its rat animal model and summary of our studies done on this model as well as the review of literature will be presented.
Keywords
Animal model for nerve repairNerve graftingSingle fascicleCable graftNerve reconstructionIntroduction
The best method to restore continuity of the transected peripheral nerve is the primary end-to-end repair [1]. In cases of direct loss or degeneration of the neural tissue due to blunt, crush, or avulsion injury, a gap is created between the nerve ends. If the gap is relatively small (does not exceed 2.5 cm), both nerve trunks can be mobilized to perform end-to-end coaptation [2]. In larger defects, stretching of the nerve ends can cause ischemia and regeneration failure, therefore the dogma is: to repair nerves without tension [3]. If direct nerve coaptation is impossible, the method of choice for nerve reconstruction is engraftment of the autologous nerve material, to replace the missing fragment.
In 1870 Philipeaux and Vulpian, the pioneers of nerve grafting, reported the first successful experimental approach [4]. Later, in 1885, Albert was the first who used nerve graft in clinic [5]. During the first and the second World War, enthusiasm for the method of nerve repair using grafts gradually decreased, as a consequence of unsatisfactory results attributed to poor surgical technique, high infection rate, and inadequate knowledge of nerve internal and topographical anatomy [6]. The resurrection of the idea of nerve grafting took place in 1947 when Seddon popularized the method of cable grafting [7]. Introduction of microsurgical techniques in 1950s by Millesi [8], compiled with the results of Suderland’s study on intraneural anatomy of the peripheral nerves [9], and revolutionized the approach for peripheral nerve grafting. Free nerve autograft repair technique became the gold standard in gap management. Despite the fact that nerve autograft technique is commonly used for over 50 years, some questions still arise. The questions are about best timing for nerve grafting, and the best method for graft coaptation. The concept of “restitutio ad integrum” was based on the restoration of the “close to normal” nerve architecture with the graft material. The cable graft technique used as a standard provided “enough” of the graft material to cover the cross section area of the severed nerve. The question, however, which has not been clearly answered, is how much of the graft material is “enough” for optimal nerve regeneration? Should the repaired nerve be fully covered with the graft, or maybe only partial coverage of cross-sectional nerve area is sufficient for the propagation of regeneration from the proximal to the distal nerve segment. This question is of great importance if we take under consideration limited sources of neural tissue accessible for harvesting, and the large amount of grafts needed in case of extensive trauma. Intrigued by the reports, that major nerves repaired with “inadequate” diameter of the graft cables resulted with surprisingly good functional recovery – we have raised the following question. Could the single fascicle of the peripheral nerve serve as a functional conduit and provide adequate nerve regeneration during nerve gap repair? This approach might also have the potential to decrease morbidity related to multiple sites of nerve harvest. To test this hypothesis we have developed rat animal model of single fascicle sciatic nerve grafting. This model enables the use of different size fascicles as grafts, covering 50 and 25 % of the total cross sectional area of the nerve stumps during gap reconstruction.
Experimental Model
All animals used in this experimental study received humane care in compliance with “Guide for the Care and Use of laboratory Animals” published by the National Institutes of Health (NIH). We used rat sciatic model of male Lewis rats, weighting 150–175 g provided by Harlan (Indianapolis, IN). The animals were caged individually and their environment was maintained at room temperature with a 12-h day/night (light/dark) cycle. Standard laboratory food for rats and water were supplied ad libitum.
Operative Technique
Surgical procedure was performed by a single surgeon using aseptic technique and microsurgical dissection under an operating microscope (ZEISS OP-MI 6-SD, Carl Zeiss, Goettingen, Germany). Animals were anesthetized by an intraperitoneal injection of pentobarbital (40 mg/kg). The sciatic nerve of each rat was exposed through a transverse skin incision and gluteal muscle-splitting dissection. Than the nerve was transected proximally and distally to acquire a 1.5 cm defect.
In order to obtain single fascicle, resected cable nerve autografts were further dissected. Under operating microscope the epineurium was gently removed using microscissors. This allowed for visualization of fascicular arrangements. Then fascicles were separated from each other in atraumatic manner using minimal blunt dissection. Special care was taken to preserve the enclosing fascicles perineurium (Fig. 54.1a–f). Thus we obtained two different size fascicles: larger single fascicle autograft matching approximately 50 % of the cross-sectional area of the transected nerve trunk, and smaller single fascicle autograft corresponding to 25 % of the cross-sectional nerve area. Coaptation was performed with a single 10-0 non-absorbable suture, based on the idea of minimal usage of foreign material. First, the epineurium of the repaired nerve was pierced inward with the needle, drawn through the interior of the nerve and pooled out from the center of the crossectional area. Next, suture was passed through the perineurium of the single fascicle graft and then back, and again from inside to outside through the epineurium of the nerve stump. Next the pull-out suture was gently tied (Fig. 54.2a–d). On the other side coaptation was performed in the identical manner. As a result autograft fascicle was precisely aligned between the nerve stumps with central orientation (Fig. 54.2e, f). Matching area was naturally greater in large when compared to small fascicle. Animals after appropriate hemostasis were sutured in a two-layer fashion (gluteal fascia and skin) using 4-0 Vicryl (Ethicon, J&J Somerville, New Jersey).
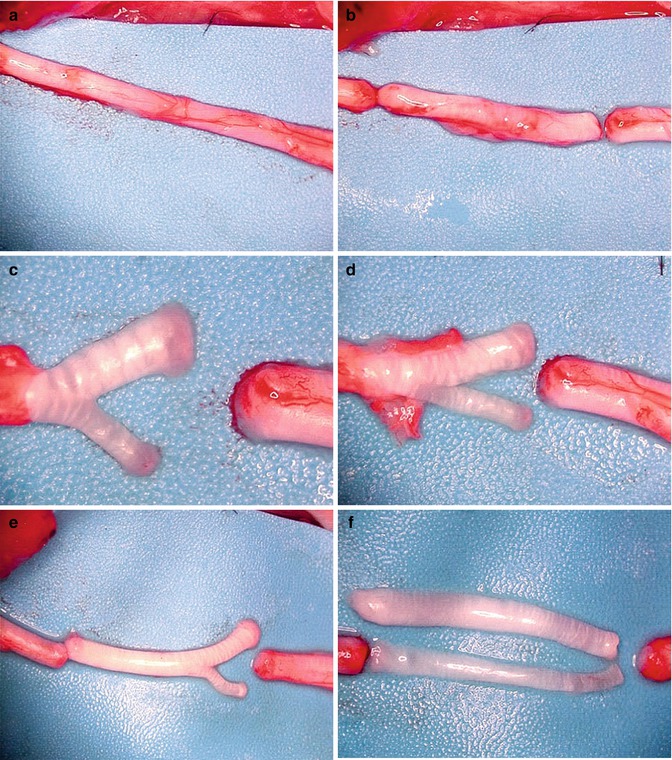
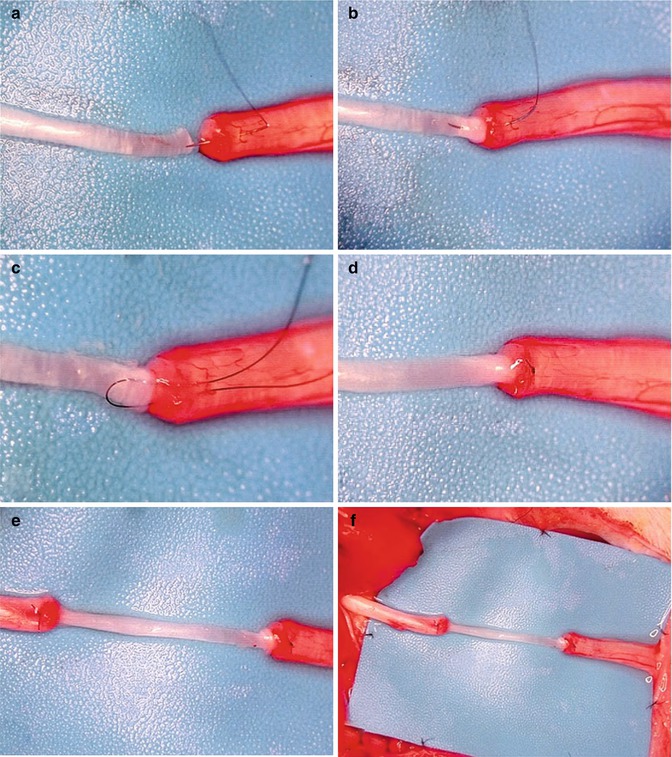

Fig. 54.1
(a–f) Microsurgical dissection of rat sciatic nerve and the preparation of single-fascicle autografts

Fig. 54.2
(a–f) The technic of the fascicle – nerve trunk coapatation using a single horizontal 10–0 mattress suture. The final result of the single fascicle graft nerve repair shows the central alignment of the graft
Summary of our Publications and the Literature Review
In our study: “The single-fascicle method of nerve grafting”, we presented the rat animal model of single fascicle autografting, as well as the functional and histological results of the nerve regeneration, while using this method of reconstruction [10




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








