Sexually Transmitted Diseases
Mary Ruth Buchness
Herbert P. Goodheart
 Anogenital warts
Anogenital warts
 Herpes simplex genitalis
Herpes simplex genitalis
 Syphilis
Syphilis
Secondary syphilis
Latent syphilis
Tertiary syphilis
Congenital syphilis
 Chancroid
Chancroid
 Lymphogranuloma venereum
Lymphogranuloma venereum
 Granuloma inguinale (donovanosis)
Granuloma inguinale (donovanosis)
Overview
Until the 1990s, sexually transmitted diseases (STDs) were commonly known as venereal diseases. Veneris is the Latin form of the name Venus, the Roman goddess of love.
Syphilis is common worldwide, and since the late 1990s infectious early syphilis has re-emerged as an important disease in western Europe and the United States and is an important facilitator of human immunodeficiency virus (HIV) transmission.
An estimated 1% of the population of the United States has clinically evident lesions of the human papilloma virus (HPV), and 15% have latent HPV infection. HPV is thought to be one of the main causes of cervical cancer, and it has also been linked with other types of cancers of the female and male reproductive system.
Herpes simplex virus (HSV) infection is another STD that presently has no cure. The incidence of HSV-2 infection is also one of the most rapidly increasing among STDs in the United States.
Chancroid is rare in the United States and Western Europe. In the United States, it is associated with the use of crack cocaine.
Lymphogranuloma venereum and granuloma inguinale are also reported rarely in the United States and Western Europe and are more frequently seen in tropical and subtropical regions.
Anogenital Warts
Basics
Anogenital warts, for the most part, are sexually transmitted viral warts caused by infection with specific types of HPV. Despite the generally benign nature of the proliferations, certain types of HPV can place patients at a high risk for anogenital cancers.
Treatment of genital warts can be difficult and lengthy. Patients should be counseled about their risk of infectivity to others. They also should be advised of their increased risk of having other STDs.
The incubation period is variable, ranging from 3 weeks to 8 months, with a reported average, in one study, of 2.8 months.
HPV has been identified in the skin of infected persons at a distance of up to 1 cm from the actual lesion; this feature may explain the high recurrence rate. HPV types 16, 18, 31 to 35, 39, 42, 48, and 51 to 54 have been identified in cervical and anogenital cancers.
The median duration of infection is 8 months. In a study of college women, only 9% had persistence of infection after 2 years, even among those infected with oncogenic subtypes.
Lesions tend to be more extensive and recalcitrant to treatment in immunocompromised persons; they also tend to grow larger and more numerous during pregnancy.
Women with HPV infection who are pregnant or who are considering pregnancy pose specific challenges. In addition to the potential for rapid proliferation of external genital warts during pregnancy, the presence of HPV infection raises concerns regarding the risk of laryngeal papillomatosis or genital HPV infections in the newborn; however, cesarean section does not eliminate the risk of transmission.
More than half of children with anogenital warts have a manifestation of viral inoculation at birth or incidental spread of cutaneous warts. Such cases often are caused by nongenital HPV infections.
Risk Factors
Transmission of anogenital HPV infection occurs largely by sexual intercourse.
Other risk factors for infection include cigarette smoking, participating in sexual activity at an early age, having a high number of sexual partners, having another STD, immunosuppression, and having an abnormal Pap smear result.
Description of Lesions
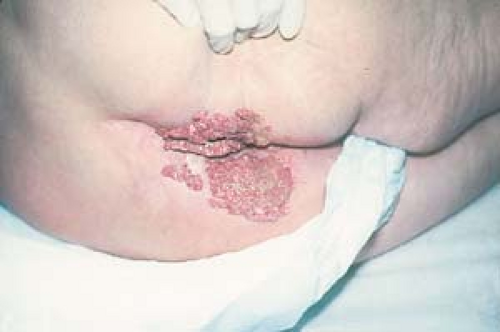
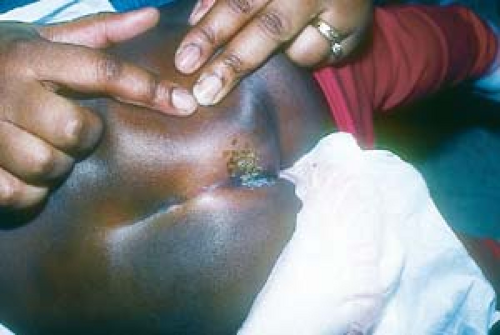
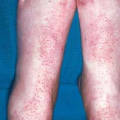
There are five morphologic types of anogenital warts, and a patient may manifest more than one type. The appearance of warts depends on its location; for example, the condyloma acuminatum type tends to occur on moist surfaces.
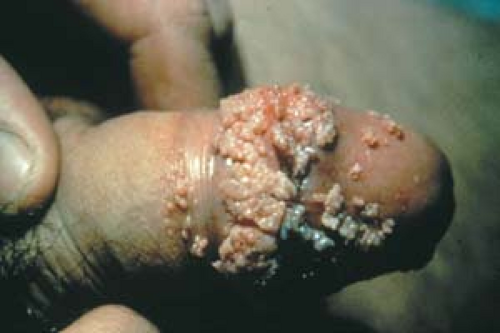
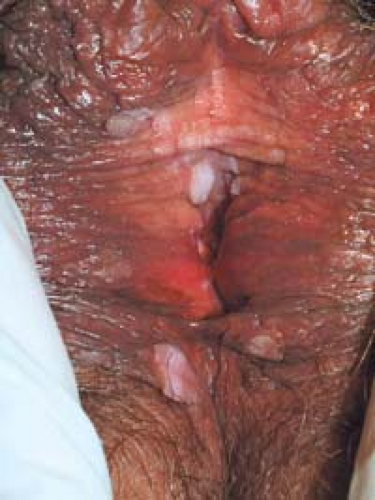
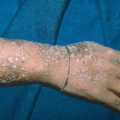
Condyloma acuminatum may resemble small cauliflowers (Fig. 19.1).
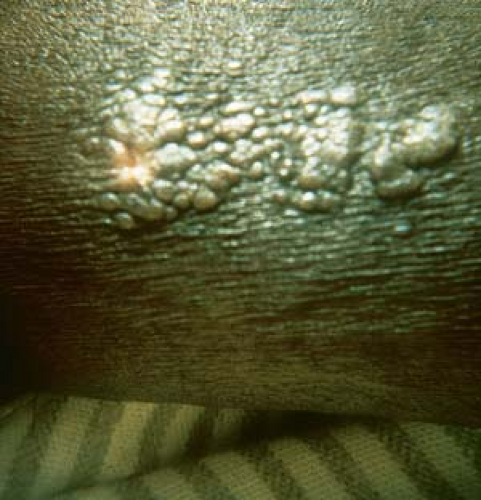
Warts may appear as smooth, dome-shaped, papular lesions (Fig. 19.2).
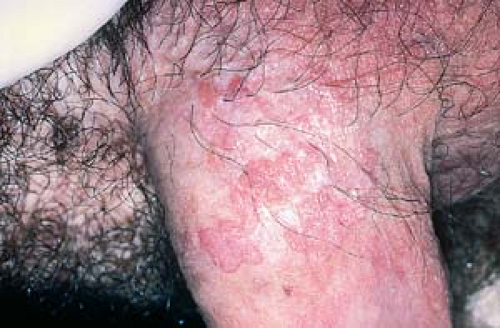
They can look like typical verrucous papules or plaques that resemble common warts (Fig. 19.3).
Occasionally, they present as flat papules that may be hyperpigmented.
Distribution of Lesions
Anogenital Lesions
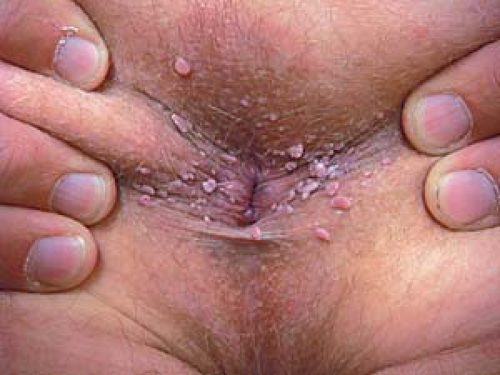
In men, lesions occur on the penis, scrotum, mons pubis, inguinal crease, and perianal area (Fig. 19.4).
In women, the vagina, labia (Fig. 19.5), mons pubis, perianal area, and uterine cervix are the most common locations.
Intra-anal warts are seen predominantly in patients who have engaged in receptive anal intercourse.
Warts may also be found in the peri- and intraurethral areas in men.
Outside the Genital Area
HPV has been associated with conjunctival, nasal, oral, and laryngeal warts.
Clinical Manifestations
Genital warts are usually asymptomatic.
Lesions may become pruritic, particularly perianal and inguinal lesions.
They may be painful or bleed if traumatized.
Genital warts may resolve spontaneously or, rarely, progress to invasive squamous cell carcinoma.
Diagnosis
The diagnosis of anogenital warts is generally straightforward when the patient presents with the typical cauliflowerlike lesions of condyloma acuminatum or with characteristic verrucous or filiform warts.
However, when lesions are papular (flat-topped), pigmented, moist, or erosive, the diagnosis may not be as clinically obvious.
Acetowhite Test on Mucous Membranes
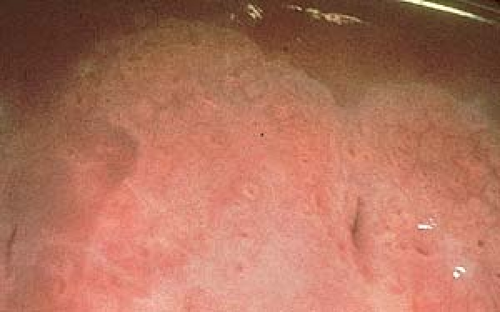
In women, colposcopy is performed using 35% acetic acid, which produces an acetowhitening of subclinical lesions on the vaginal and cervical mucosa (Fig. 19.6).
Atypia or koilocytosis found on Pap smears represents early changes resulting from HPV infection.
Acetowhite Test on Non-Mucous Membrane Areas
Application of a 5% concentration of acetic acid for 15 to 20 minutes makes subclinical lesions turn white.
Any lesion with epidermal hyperkeratosis appears white. Thus, this method often produces false-positive results and is no longer recommended for routine screening for genital warts.
Biopsy
A biopsy may be needed to identify confusing anogenital lesions.
After local anesthesia with lidocaine, curved iris scissors are used to obtain a small specimen (snip biopsy) from the labia minora or perianal area. A punch biopsy or, more simply, a shave biopsy may be obtained from non-mucous membrane skin (see Chapter 26, “Diagnostic and Therapeutic Procedures”). If an ulcer or an indurated nodule is present—particularly if carcinoma is suspected—a punch or excisional biopsy should be performed.
A biopsy is used to rule out anogenital bowenoid papulosis or frank squamous cell carcinoma in atypical or recalcitrant lesions.
Normal Anatomic Structures
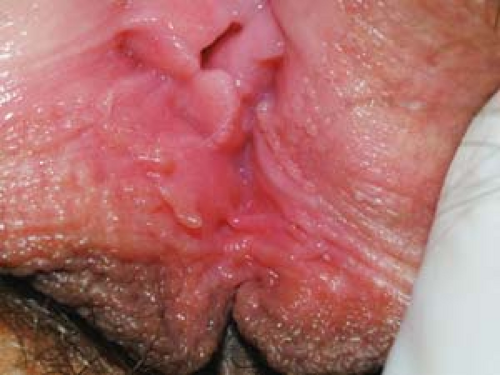
In women, vestibular papillae are normal anatomic structures. Unlike warts, vestibular papillae (vulvar papillomatosis) occur near the vaginal vestibule in symmetric clusters or in a linear pattern. They often appear as monotonous, small, smooth projections that resemble cobblestones (Fig. 19.7).
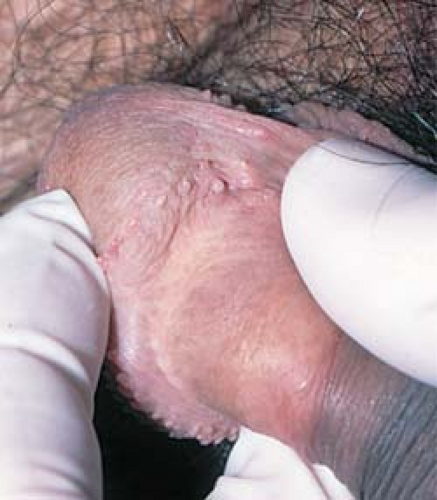
In men, pearly penile papules are frequently mistaken for warts. They are small, skin-colored to shiny, pearly papules that are located around the rim of the corona of the glans penis (Figs. 19.8 and 19.9).
Fordyce spots are angiokeratomas. They occur on the medial labia minora in many women (see Fig. 21.47).
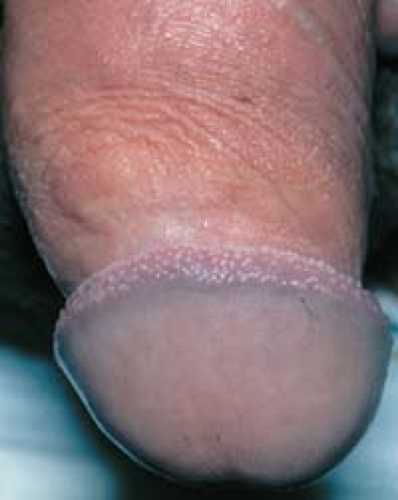 19.8 Pearly penile papules. These normal anatomic structures occur as shiny papules that are present around the corona of the glans penis and the frenum of the penis. |
Benign Lesions
Common benign skin lesions, such as skin tags, seborrheic keratoses, and melanocytic nevi, may also be easily mistaken for warts.
Skin tags are smooth and may be pigmented or skin-colored. Seborrheic keratoses and melanocytic nevi often have a verrucous (keratotic) appearance and may be pigmented.
Other Conditions
Hemorrhoids. Not infrequently, anal hemorrhoids are mistaken for warts. Hemorrhoids are smooth and compressible.
Molluscum contagiosum. This pox virus infection can easily be confused with, and may coexist with, genital warts. It is seen most often in young children and in patients with HIV infection and in sexually active young adults (see Chapter 6, “Superficial Viral Infections,” and Chapter 24, “Cutaneous Manifestations of HIV Infection”). The lesions are dome-shaped, waxy or pearly white papules with a central white core, which is often revealed by inspection with a handheld magnifier.
Condyloma latum of secondary syphilis. Lesions are moist, smooth-surfaced, and, usually, whitish and flat-topped. Serologic tests for syphilis are positive (see Fig. 19.20).
Malignant Neoplasms
When any of the following conditions are suspected, a biopsy should be performed.
Bowenoid papulosis. These lesions are clinically similar to, and often indistinguishable from, flat or dome-shaped genital warts. They are associated with HPV type 16 or 18. Histologically, bowenoid papulosis demonstrates squamous cell carcinoma in situ; however, it follows a largely benign clinical course.
Giant condyloma acuminatum. Also known as a Buschke-Löwenstein tumor, this lesion is a low-grade, locally invasive squamous cell carcinoma that can arise from and appear as a fungating condyloma (Fig. 19.10). It is associated with HPV types 6 and 11 and should be considered in the differential of lesions measuring greater than 1 cm in diameter. Only radical surgical extirpation is considered appropriate treatment.
Squamous cell carcinoma. These lesions are rapidly growing nodules or tumors, and they may be erosive or ulcerative.
Counseling
Patients should be advised about the long latency period of HPV; thus, a patient may not have contracted condyloma from his or her current partner.
Male patients should use condoms at least 1 year after clinical infection is treated; however, condoms are not perfect protection because warts can occur on genital areas other than the penis or vagina.
In affected women, there is a risk of malignant degeneration to cervical intraepithelial neoplasia or squamous cell carcinoma. If cervical warts are found during examination or if vulvar neoplasia is confirmed by biopsy, referral for colposcopic evaluation is indicated.
It is recommended that anogenital warts be treated in pregnant women during the second and third trimesters and that vaginal delivery be performed if possible.
In affected men with perianal warts, there is a risk of malignant degeneration to anal intraepithelial neoplasia or anal carcinoma. Atypical lesions should be biopsied.
The U.S. Centers for Disease Control and Prevention (CDC) recommends cesarean section only when the vaginal outlet is obstructed by extensive condylomata or if vaginal delivery would cause excessive bleeding.
Patients who have internal anal or rectal warts tend to have continual recurrences of external warts and should be referred to a rectal surgeon.
Diagnosis of genital warts in a child requires that the clinician report suspected abuse to begin an evaluation process that may or may not confirm sexual abuse (Fig. 19.11).
Surgical Therapy
Cryosurgery with liquid nitrogen. Cryosurgery is very effective for treating multiple, small warts (e.g., lesions on the shaft of the penis and vulva). Cryotherapy is also safe for the mother and fetus when used during the second and third trimesters of pregnancy.
Electrodesiccation and curettage. This is quite effective for a limited number of lesions on the shaft of the penis.
Carbon dioxide laser treatment. This is more expensive and includes a danger of the operator developing laryngeal lesions from virus in the laser plume.
Surgical excision (useful for debulking large “cauliflower” lesions). Large, unresponsive lesions around the rectum or vulva can be treated with scissor excision of the bulk of the mass followed by electrocautery of the remaining tissue down to the skin surface.
Intralesional Therapy
Interferon-α2b (11.5 units three times weekly for 3 weeks). This is not recommended by the CDC because of high expense and a lack of increased efficacy over other treatments. It may also cause flulike symptoms.
Vaccine
Quadrivalent vaccine (Gardasil) protects against HPV subtypes 6, 11, 16, and 18.
A bivalent vaccine protects against subtypes 16 and 18. Approval is pending.
The Advisory Committee on Immunization Practices of the CDC recommends routine immunization of girls at 11 and 12 years of age, with the vaccine being made available to girls as young as 9 and women as old as 26. Efficacy has not been studied in women older than 26 or in males.
Women younger than 26 years of age with abnormal or equivocal Pap smears, known HPV infection, or genital warts can be vaccinated. However, the vaccine does not protect against HPV subtypes that the vaccine recipient already has.
Gardasil can be used in women younger than 26 years of age who are breastfeeding or are immunocompromised; however, those women who are immunodeficient may not produce an adequate number of antibodies in response to the vaccine.
Pregnant women should not be vaccinated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree