Introduction
In the past two decades, significant advances have been made in the description of the sensory anatomy of the head and neck, mostly in the cosmetic surgery literature. This progress was driven in large part by the discovery that compression of sensory nerves in the head and neck may contribute to the pathogenesis of migraine headaches.
Migraine headaches are a robust clinical challenge that affects 17.1% of women and 5.6% of men in the United States.1 Traditional pharmacologic treatment is often insufficient. New surgical options for the treatment of migraine headache have been developed based on the finding that extracranial sensory branches of the trigeminal and cervical spinal nerves can be irritated, entrapped, or compressed at multiple points along their anatomical course, ultimately leading to a cascade of physiologic events that results in migraine headaches.2–5
In addition to the abundant clinical studies that have been published in support of the surgical treatment of migraine headaches, complementary anatomical studies have been performed that describe the detailed anatomy of the sensory nerves involved, as well as the compression points along their course. These include the frontal trigger point (supraorbital and supratrochlear nerves, or STNs), the temporal trigger point (zygomaticotemporal nerve [ZTN] and auriculotemporal nerve [ATN]), the occipital trigger point (greater occipital, third occipital, and lesser occipital nerves [LONs]), and the nasoseptal trigger point. In this chapter, we begin by summarizing the hypotheses on the pathogenesis of migraine headaches and then describe the detailed anatomy of the sensory nerves involved, along with their compression points.
Pathogenesis of Migraine Headaches
The final common pathway in the pathogenesis of migraine headaches appears to be hyperexcitability of cerebral neurons resulting from lower than normal threshold to excitation of the neuronal membrane,6 which is believed to be due to localized dural inflammation and vasodilation of meningeal vessels that are supplied by the trigeminal nerve. The mechanism of aura is believed to be cortical spreading depression, characterized by cortical neuronal excitation, followed by depression of normal neuronal activity.6
Irritation of the trigeminal nerve, from either a central7 or peripheral source, causes inflammation and vasodilation in the region of the dura mater supplied by the trigeminal nerve via release of nociceptive mediators such as calcitonin gene-related peptide, substance P, and neurokinin A.6,8 The central trigger theory of migraine headache postulates that central neurovascular events cause irritation of the trigeminal nerve, leading to the release of nociceptive substances from the nerve, triggering dural inflammation and the migraine pain cascade. This theory ascribes the proven ability of botulinum toxin A to reduce the frequency and severity of migraine headaches9,10 to its ability to be taken up by the trigeminal nerve peripherally, travel downstream through the axon, and block the release of nociceptive substances at the synaptic interface of the trigeminal nerve with the dura. In an in vitro study, Durham et al found that botulinum toxin A decreased the amount of calcitonin gene-related peptide released from activated rat trigeminal neurons.11
In contrast, the peripheral trigger theory of migraine headache postulates that irritation of the trigeminal nerve occurs peripherally via compression of one of the sensory branches of the trigeminal or cervical nerves by muscle, fascia, bone, artery, or mucosa. This theory attributes the efficacy of botulinum toxin A to its ability to weaken temporarily the muscles by blocking acetylcholine release at the neuromuscular junction, thus reducing muscular compression of branches of the trigeminal nerve.12 This theory is also validated by the efficacy of surgical trigger-point decompression. Indeed, surgical release of branches of the trigeminal nerve entrapped in muscle, fascia, bone, artery, and mucosa has been shown to be effective at reducing the frequency, intensity, and severity of migraine headaches in most patients who have been refractory to medical management through retrospective chart reviews,5,13 prospective cohort studies,2,3 and a prospective randomized trial using sham surgery as a placebo control.4 In addition, patients with migraine headaches often have tenderness to palpation that precisely localizes to their trigger points, which further lends credence to the peripheral trigger theory.14
The central and peripheral theories of the pathogenesis of migraine headaches may not, in fact, be incompatible. A combination of peripheral and central sensitization may act in synergy to produce migraine headaches,15 and current therapeutic strategies—including medications, botulinum, and surgical decompression—may prove to be multimodal in their mechanism of action.
Peripheral Trigger Points in Migraine Headaches
Frontal Trigger Point
Supraorbital Nerve
Origin and Course
The supraorbital nerve (SON) is one of the two terminal cutaneous branches of the frontal nerve, which is a branch of the ophthalmic division of the trigeminal nerve (V1). The frontal nerve passes through the superior orbital fissure and divides into two branches: the SSTN and the SON, both of which run beneath the orbital roof. The SON proceeds laterally and most commonly exits the orbit through a supraorbital notch located on the supraorbital rim, but it can also exit through a foramen located cephalad to the supraorbital rim.
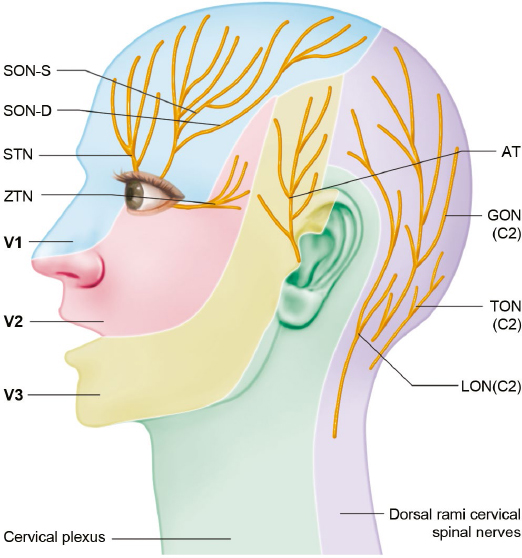
Fig. 10.1 Sensory distributions of nerves involved in migraine trigger points: AT, auriculotemporal nerve; GON, greater occipital nerve; LON, lesser occipital nerve; SON-D, deep branch of the supraorbital nerve; STN, supratrochlear nerve; TON, third occipital nerve; SON-S, superficial branch of the supraorbital nerve; V1, ophthalmic branch of the trigeminal nerve; V2, maxillary branch of the trigeminal nerve; V3, mandibular branch of the trigeminal nerve; ZTN, zygomaticotemporal nerve.
After exiting the orbit, the SON divides into a deep (lateral) branch and a superficial (medial) branch. The deep branch has a more consistent course and runs between the galea aponeurotica and the periosteum toward the temporal fusion line laterally16 and provides sensation to the frontoparietal scalp (Fig. 10.1). Cuzalina and Holmes described the reproducible location of the deep branch of the SON in a study that examined 75 patients undergoing endoscopic browlift17 and found the deep branch of the SON to be located an average of 0.56 mm from a vertical line drawn tangentially to the medial limbus of the iris. The superficial branch of the SON is more variable in location. It pierces the frontalis muscle in a fanlike pattern with numerous branches and provides sensory innervation to the forehead skin and anterior scalp.16
Points of Compression and External Landmarks
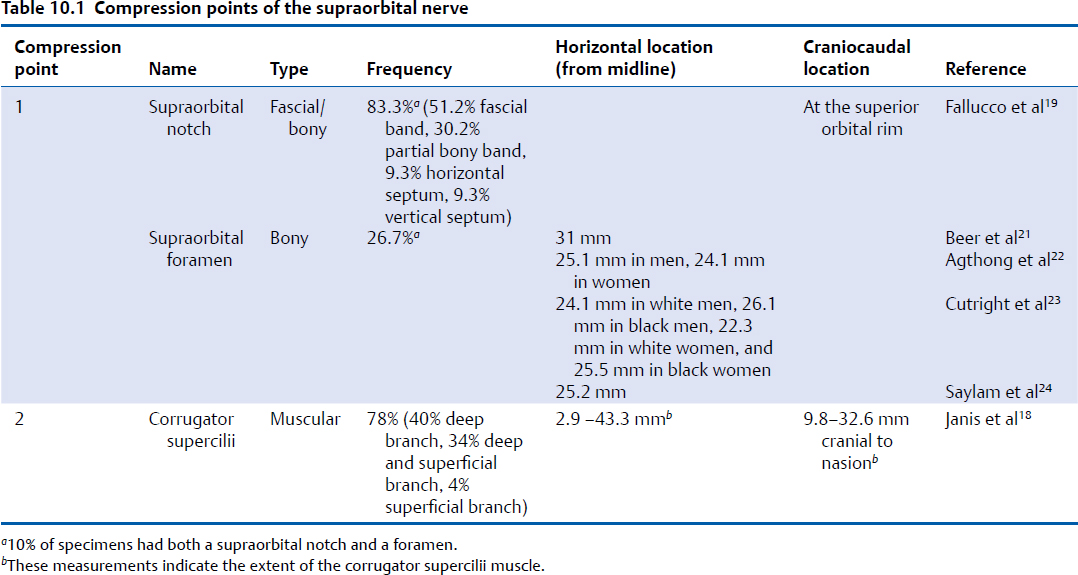
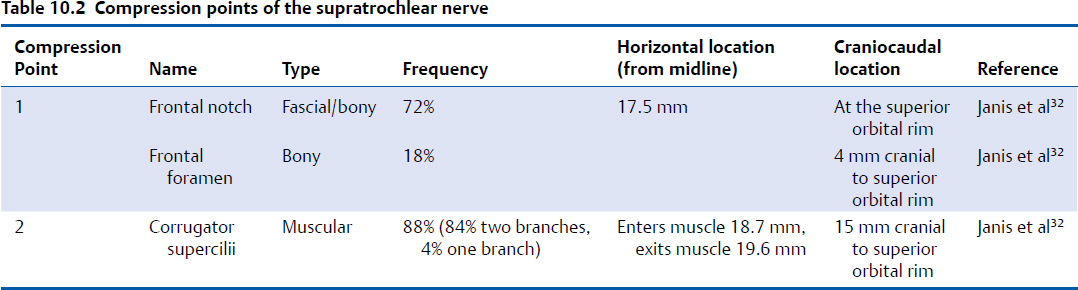
The first compression point of the SON consists of either the supraorbital notch or foramen (Fig. 10.2, Table 10.1). When a supraorbital notch is present as the SON exits from the superior orbital rim, there is frequently a fascial band that completes the circular shape of the notch and can compress the SON against the frontal bone, as observed by Janis et al18 and then studied extensively by Fallucco et al,19 who found that a supraorbital notch was present 83% of the time and a foramen 27% of the time (10% of specimens had both a notch and a foramen). They found that 86% of supraorbital notches had a fascial band. The fascial bands were further divided into three classifications. Type 1 bands, which occurred in 51.2% of specimens, were described as “simple” and consisted of a single fascial band. Type 2 bands, occurring in 30.2% of specimens, consisted of bony spicules with a fascial band completing the bridge overlying the supraorbital notch. Type 3 bands, occurring in 18.6% of specimens, contained a septum that allowed for more than a single passageway for the neurovascular bundle through the supraorbital notch.
These were further divided into types 3A and 3B classification, depending on whether the septum was horizontal or vertical, with each occurring 9.3% of the time (Fig. 10.2).19 When present, a supraorbital foramen can act as a bony compression point.20 Beer et al described the SON exit from 507 European skulls and discovered that in 74% of cases, the location of the exit was asymmetric between sides in the same person.21 The average distance from the nasion to either a supraorbital notch or foramen was 31 mm. A single exit point exists in most circumstances, but in approximately 10 to 15% of people, more than one exit point exists.21,22 Agthong et al examined specimens from 70 men and 40 women in an Asian population and noted that when measured from the midline, the nerve exit trended toward a more lateral location in men (25.1 mm) than in women (24.1 mm).22 Interestingly, this study demonstrated an equal rate of supraorbital notch versus foramen in this population. Conversely, Cutright et al examined 20 specimens each from white versus black and male versus female populations and found that a notch was present 92.5% of the time and a foramen present in the remaining specimens.23 They also noted a more lateral location in men versus women and in blacks versus whites (24.1 mm in white men, 26.1 mm in black men, 22.3 mm in white women, and 25.5 mm in black women). Saylam et al described the presence of a supraorbital notch in 71.6% of specimens, with an average distance of 25.2 mm from the midline.24 Webster et al examined the variability in nerve exit patterns between sides within the same person in a study with 111 skulls.25 In approximately 50% of specimens, a bilateral supraorbital notch was found, in 25% a bilateral supraorbital foramen, and in 25%, a notch on one side with a foramen on the contralateral side. Extrapolating definitive conclusions about the presence of a notch versus a foramen as well as a precise location of nerve exit from the data reviewed here is limited by the fact that no two studies examined comparable populations in sufficient numbers with comparable reference points to measure nerve exit; however, the data highlight the importance of appreciating the frequent variability within different people and even within the same patient.
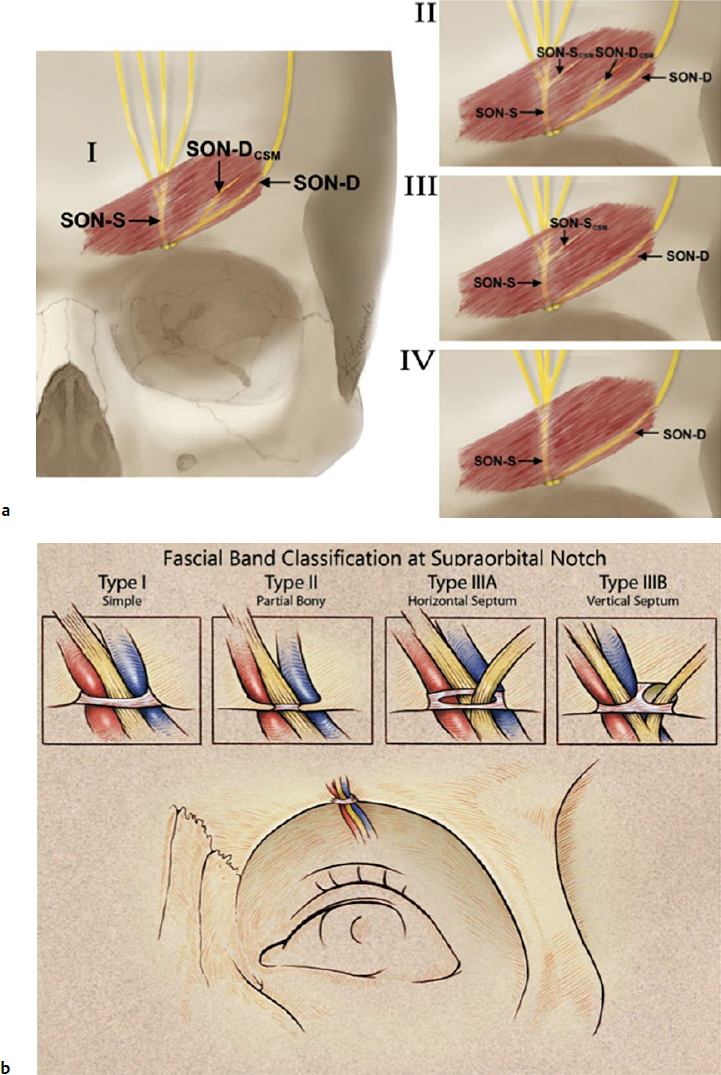
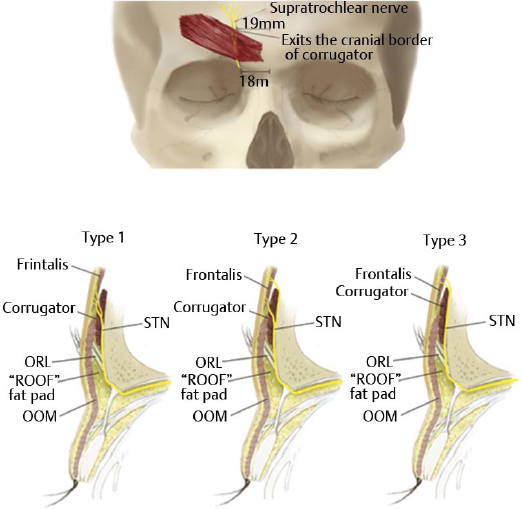
The second compression point of the SON is the corrugator supercilii muscle (CSM), where branches of the nerve course directly through the muscle in 78% of people.18,26 In an anatomical study of 25 cadavers, Janis et al described the branching patterns of the SON in relation to the CSM and discovered four unique patterns.18 In a type I branching pattern, which occurred in 40% of specimens, the deep branch of the SON interacted with the CSM. In a type 2 pattern, occurring in 34% of specimens, branches of both the superficial and deep branches of the SON interacted with the CSM. In a type 3 pattern, which occurred in 4% of specimens, only the superficial branch of the SON interacted with the CSM. Lastly, in a type IV pattern, occurring in 22% of specimens, the branching of the SON occurred more cephalad to the CSM, without any interaction with the muscle (Fig. 10.2, lower left).
Fig. 10.2 (a) Compression points of the supraorbital nerve. (Reproduced with permission from Bindingnavele VK1, Bresnick SD, Urata MM, et al. Superior results using the islandized hemipalatal flap in palatoplasty: experience with 500 cases. Reproduced from Plast Reconstr Surg 2008; 122(1):232.) (b) Supraorbital nerve course through the corrugator supercilii muscle. Fascial band classification at the supraorbital notch. (Reproduced with permission from Fallucco M1, Janis JE, Hagan RR. The anatomical morphology of the supraorbital notch: clinical relevance to the surgical treatment of migraine headaches. (Reproduced with permission from Plast Reconstr Surg 2012;130(6):1227–1233.)
Clinical Correlation
The supraorbital and STNs constitute the frontal trigger point, the most commonly described trigger point among migraine patients. Patients with a frontal trigger point typically have a strong CSM, as indicated by deep frown lines. They often have tenderness over the SON, and they experience headaches that are “imploding” and are worse in the afternoon and with stress.27
The first compression point is decompressed via a supraorbital foraminotomy (for a foramen) or fasciotomy (for a notch). The second trigger point is addressed by resection of the corrugator myofascial unit through either subtotal resection of the CSMs or more effectively by resection of the entire glabellar muscle group, including the CSM and portions of the depressor supercilii and procerus muscles. This resection is achieved through either a transpalpebral approach or with an endoscopic approach through small hairline incisions,2 although the endoscopic approach has been shown to visualize more of the muscle and therefore may lead to more successful complete resection and better decompression.28,29
Multiple clinical studies have been completed that address muscular, fascial or bony release of the frontal trigger point.5,13,30 Success rates of migraine headache improvement or migraine headache elimination have been high when performed independently or concurrently with decompression of other trigger points. Interestingly, the proportion of patients who benefit from surgical decompression (79.2%)5 closely mirrors the percentage of patients who have interaction between the SON and the CSM (78%).18
Supratrochlear Nerve
Origin and Course
The STN is one of the two terminal cutaneous branches of the frontal branch of the ophthalmic division of the trigeminal nerve (V1). It provides sensory innervation to the midline forehead (Fig. 10.1). Much less has been published about the anatomy of the smaller STN compared with the larger SON. The STN proceeds medially to exit the superior orbital rim via a notch or a foramen, then travels cranially through the CSM. Miller et al examined the anatomy of the STN in 10 cadavers and found that the nerve coursed between 1.6 and 2.3 cm lateral to the midline at the level of the superior orbital rim.31 After exiting the orbital rim, the STN courses through the CSM as it travels cephalad.
Points of Compression and External Landmarks
The first compression point of the STN occurs as it exits the orbit via either a notch or a foramen (Table 10.2). In a dissection of 50 cadaver hemiheads by Janis et al, the STN was found to exit the orbit via a notch in 72% of specimens, located an average of 1.75 cm from the midline.32 The floor of the notch consisted of a fibrous band in all cases. In 68% of specimens, the nerve was observed to pass directly through the notch; however, in 8%, the nerve pierced the fascial band and coursed directly through the connective tissue. In another 8%, the fascial band was very broad and flattened the nerve against the bony surroundings. A true bony foramen occurred in 18% of specimens and was located an average of 4 mm cranial to the superior orbital rim.
The second compression point occurs as the STN interacts with the CSM. Janis et al found that 84% of the time, the STN divided into two branches within the retro-orbicularis fat, which then both entered the CSM at an average of 18.7 mm lateral to the midline, and exited it 19.6 mm lateral to the midline and 15 mm cranial to the superior orbital rim (Fig. 10.3).32 In 4% of specimens, only one STN branch entered the CSM, and the other one stayed deep; in 12% of specimens, neither branch traveled through the CSM, but rather both remained deep to the muscle.
Clinical Correlation
The STN and the SON constitute the frontal trigger point, and release of both nerves is usually achieved simultaneously. Anatomical studies involving the STN have highlighted the importance of extending surgical dissection medially to fully decompress it. It has been hypothesized that failure to fully decompress the most medial aspect of the frontal trigger site may have resulted in early clinical failures.32
Temporal Trigger Point
Zygomaticotemporal Nerve
Origin and Course
The ZTN is one of the two terminal branches of the zygomatic nerve, which is a branch of the maxillary division of the trigeminal nerve (V2).33 The zygomatic nerve enters the orbit via the inferior orbital fissure, travels along the lateral orbital wall,34 then divides into the zygomaticofacial nerve (ZFN) and ZTN. The ZTN provides sensation to the skin of the temple (Fig. 10.1), as well as parasympathetic innervation to the lacrimal gland.34
In a study by Janis et al, 30% of subjects were found to have two ZTN branches.33 Among those, 20% had branching of the ZTN within the orbit, with the two branches exiting via two separate zygomaticotemporal foramina. In the remaining 80%, the ZTN branched after exiting the orbit. Accessory branches of the ZTN were found in 50 to 55% of patients. Among those with accessory ZTN branches, the ZTN branches were cranial to the main branch in 30% on the left (located an average of 16mm lateral and 12.2 mm cranial to the lateral palpebral commissure) and in 55% on the right (located an average of 15.7 mm lateral and 16.5 mm cranial to the lateral palpebral commissure). The ZTN branches were immediately adjacent to the main branch in 30% on the left (located an average of 17.7 mm lateral and 6 mm cranial to the lateral palpebral fissure) and in 9% on the right (located an average of 19.0 mm lateral and 5.0 mm cranial to the lateral palpebral commissure). The ZTN branches were lateral to the main branch in 40%, on the left (located an average of 34.2 mm lateral and 6.7 mm cranial to the lateral palpebral commissure), and in 36% on the right (located an average of 28.7 mm lateral and 6.0 mm cranial to the lateral palpebral commissure). In all cases with a lateral branch, this branch travelled horizontally to join the ATN. Tubbs et al have confirmed this communication with the ATN in 13% of hemiheads.35 In addition, Odobescu et al found that 82% of individuals had small connections between the ZTN and the frontal division of the facial nerve.36
Points of Compression and External Landmarks
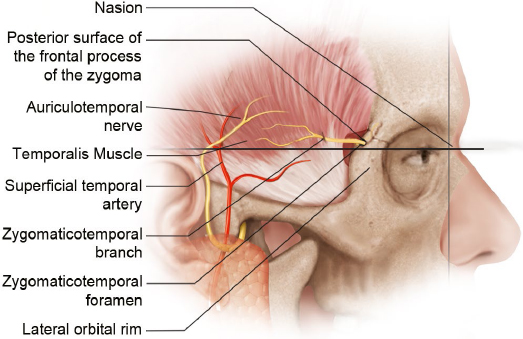
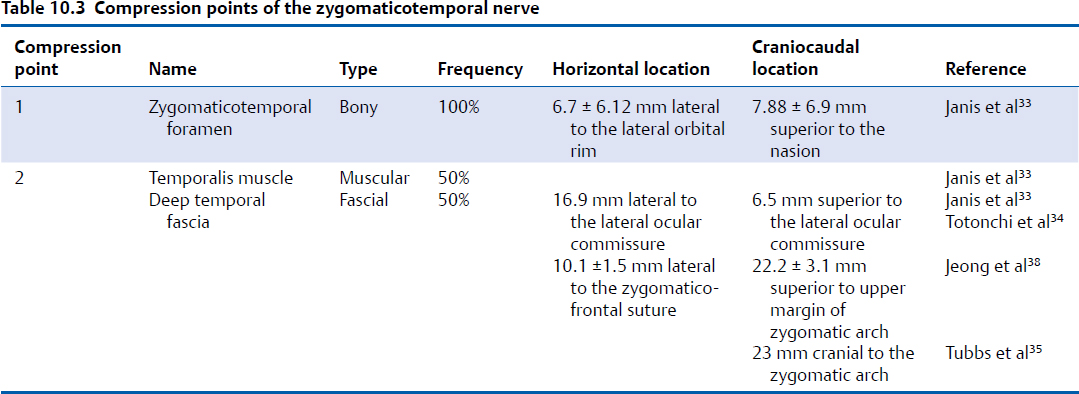
The first compression point occurs as the ZTN enters the temporal fossa. The nerve exits the lateral orbit via a bony canal and emerges in the temporal fossa via a bony foramen (Fig. 10.4, Table 10.3)34; 94% of individuals have one zygomaticotemporal foramen, and the remaining 6% have two foramina.33 The foramen is located in the frontal bone within the temporal fossa and is found an average of 6.7 ± 6.12 mm lateral to the lateral orbital rim and 7.88 ± 6.9 mm cranial to the nasion. In an anatomical study of 400 hemiskulls, Loukas et al found that the location of the zygomaticotemporal foramen varied widely depending on ethnic background and that up to 50% of individuals lacked a zygomaticotemporal foramen.37
The second compression point is the temporalis muscle/deep temporal fascia. After exiting the orbit, the ZTN enters the deep aspect temporalis muscle and travels intramuscularly in 50% of individuals.33 Among those whose ZTN has an intramuscular course, this course is short and direct in 44% and long and tortuous in 56%. In the 50% of individuals who have no intramuscular course, the ZTN travels between the temporal periosteum and the temporalis muscle before piercing the deep temporal fascia. In an intraoperative endoscopic anatomical study of 20 patients, Totonchi et al found that the ZTN pierced the deep temporal fascia 16.9 mm lateral and 6.5 mm cranial to the lateral palpebral commissure,34 whereas Jeong et al found that it pierced the deep temporal fascia 10.1 ± 1.5 mm lateral to the zygomaticofrontal suture and 22.2 ± 3.1 mm cranial to the upper margin of the zygomatic arch.38 This was confirmed by Tubbs et al, who found that the ZTN pierced the deep temporal fascia an average of 23 mm cranial to the zygomatic arch.35
Clinical Correlation
Patients with migraine headaches originating from the ZTN tend to have temporal pain, usually in the morning, associated with stress, grinding, clenching, or temporomandibular joint dysfunction.27 Murillo first described open resection of the ZTN and of the superficial temporal artery (STA) for temporal migraine headaches in 34 patients in 196839; results were positive in 88.2% of patients. The technique for ZTN decompression has since been refined by Guyuron and is now usually performed via an endoscopic approach. Dissection is performed just superficial to the deep temporal fascia until the ZTN is identified. It is then avulsed, with resection of approximately 3 cm of the nerve, allowing the proximal end of the nerve to retract and get buried into the temporalis muscle.40 Avulsion of the ZTN may cause temporary paresthesia and anesthesia in the temporal region, which are usually temporary.41 The method of ZTN decompression has been examined by Chim et al.42 In an animal study on rat sural nerves, they found that nerve avulsion and burying in muscle led to the lowest rate of neuroma formation.
In a study of 246 patients undergoing endoscopic decompression of the ZTN, Kurlander et al found that at 1 year postoperatively 55% of patients had complete elimination of temporal migraine headaches, with an additional 30% of patients having significant improvement.41 In a prospective evaluation of 71 patients with a temporal trigger site undergoing surgical avulsion of the ZTN, with a mean 396-day follow-up, Guyuron et al demonstrated complete migraine elimination in 63%, with at least 50% improvement in migraine severity, duration, and frequency in an additional 35%.3 In a single-blinded, randomized controlled trial comparing actual decompression of the ZTN in 19 patients with sham surgery in nine patients, those undergoing actual decompression had significant improvements in migraine intensity, frequency, and duration from baseline, which was not the case for those undergoing sham surgery.4 In a review of 19 patients undergoing ZTN avulsion for the treatment of migraine headaches from a temporal trigger site, with a mean follow-up of 661 days, Janis et al demonstrated complete migraine elimination in 52.6% of patients, with an additional 36.8% having at least 50% improvement.5
Auriculotemporal Nerve
Origin and Course
The ATN is a branch of the mandibular division of the trigeminal nerve (V3). It is a sensory nerve that provides sensation to the tragus and anterior ear, as well as to the posterior temporal region (Fig. 10.1). It also carries autonomic nervous fibers, providing sympathetic innervation to the scalp and parasympathetic innervation to the parotid gland.
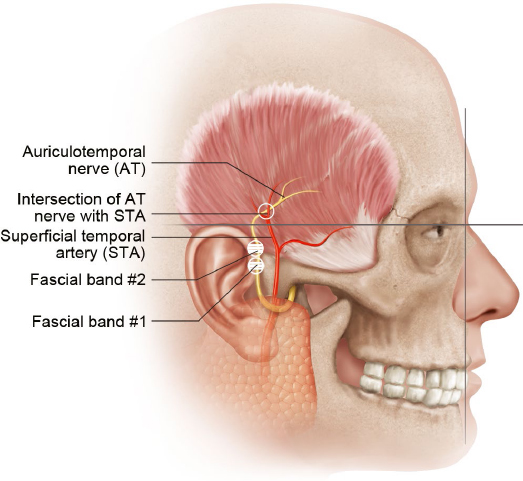
The ATN emerges within the superficial parotid gland along the posteromedial aspect of the temporomandibular joint43 and travels cranially within the temporoparietal fascia, crossing the posterior aspect of the zygomatic arch.44 It travels as a single branch in 50% of people, and up to four branches in the remainder.45 As the nerve travels cephalad, it parallels and runs lateral to the STA.44 In the upper temporal area, the nerve becomes more superficial, lying superficial to the temporoparietal fascia.
Points of Compression and External Landmarks
In an anatomical study of 10 cadavers, Chim et al found that in all specimens the ATN traveled under a fascial compression band (compression point 1), present an average of 13.1 ± 5.9 mm anterior and 5.0 ± 7.0 mm cranial to the most anterosuperior point of the external acoustic meatus (Fig. 10.5, Table 10.4).46
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree