14 Salivary gland tumors
Synopsis
 Salivary gland tumors occur in the range of 1–3 per 100 000 in the US.1
Salivary gland tumors occur in the range of 1–3 per 100 000 in the US.1
 Diagnosis of an enlarged salivary gland may be difficult and requires methodical examination and appropriate testing.
Diagnosis of an enlarged salivary gland may be difficult and requires methodical examination and appropriate testing.
 Salivary gland tumors can be classified by their cell type and behavior pattern.
Salivary gland tumors can be classified by their cell type and behavior pattern.
 Treatment decisions are based on the lesion’s natural history.
Treatment decisions are based on the lesion’s natural history.
 Prognosis is variable and depends upon tumor type and stage at diagnosis.
Prognosis is variable and depends upon tumor type and stage at diagnosis.
Introduction
1. Most parotid tumors are benign, while submandibular and sublingual tumors are more likely to be malignant.
2. Fine-needle aspiration (FNA) is very useful in the preoperative diagnosis of the tumor masses.
3. Techetium scans are very effective in the diagnosis of Warthin’s tumors.
4. High-grade mucoepidermoid and squamous cell carcinomas have a very high incidence of nodal metastases.
5. Knowledge of the anatomy and variations of the branches of the facial nerve is critical to the safe resection of the parotid gland.
Basic science/disease process
Anatomy
Parotid gland
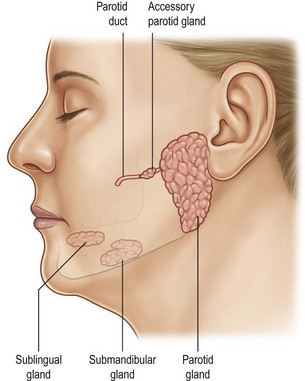
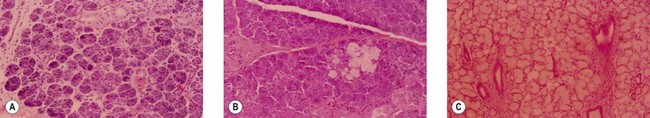
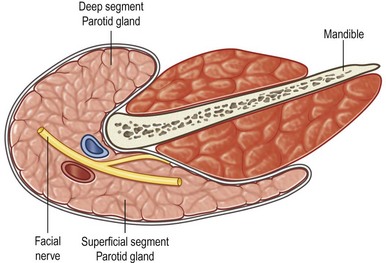
The parotid gland is the largest of the salivary glands and is located on the face between the zygomatic arch and the angle of the mandible (Fig. 14.1). Histologically, the parotid is virtually totally serous glands (Fig. 14.2A). Although the parotid has a dense sheath overlying its surface, which is derived from the submuscular aponeurotic system (SMAS), it is not an encapsulated gland. It has multiple small segments, growths, and islands of tissue that are found within the subcutaneous tissue of the face. Furthermore, the parotid gland is not segmented into “lobes.” Rather it is a single-lobed, C-shaped gland wrapping itself from its location over the mandible, around the ascending ramus, and to a location deep to the ramus, which is called the “deeper segment” (Fig. 14.3). The portion of the gland overlying the mandible is separated by the plane made by the branches of the facial nerve coursing within the gland, hence the “superficial segment” overlying the facial nerve.
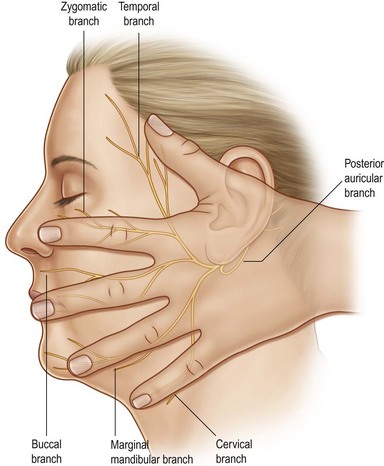
The facial nerve exits the skull at the stylomastoid foramen, posterior to the styloid process, and gives off its first branch, the posterior auricular nerve. This nerve innervates the auricularis muscle (allowing some patients to wiggle their ears), and gives a branch to the posterior belly of the digastric muscle (the anterior belly is innervated by a branch of the trigeminal nerve), and another branch to the stylohyoid muscle. The remainder of the main trunk of the facial nerve penetrates the mass of the parotid gland to arborize into its five remaining branches (Fig. 14.4):
1. temporal branch to the frontalis muscle
2. zygomatic branch to the orbicularis oculi muscle
3. buccal branch to the muscles of the cheek and upper lip
4. mandibular branch to the muscles of the lower lip and chin
The main secretory duct of the gland, the Stensen’s duct, courses through the structures of the cheek to empty into the oral cavity through its orifice in the buccal mucosa at the level of the crown of the upper first and second molar teeth (Fig. 14.1). The course of this duct through the cheek is along an imaginary line drawn from the tragus to the curve of the lateral nares. Occasionally, a small “accessory” parotid gland may become enlarged, presenting as a small nubbin of tissue anterior to the parotid gland, and overlying the Stensen’s duct of the parotid.
Submandibular gland
The submandibular glands (also called the submaxillary glands) lie bilaterally deep to the horizontal body of the mandible, and are about one-fourth to one-third the size of the parotid gland (Fig. 14.1). They secrete saliva through the left and right Wharton’s ducts, which course under the lateral floor of the mouth, each of which exits into the oral cavity just short of the midline along the root of the undersurface of the tongue. On histologic evaluation, the submandibular gland is composed of a mixture of serous and mucous glands (Fig. 14.2B).
Sublingual gland
This gland is the smallest of the salivary glands, and is frequently half the size of the submandibular gland. It is located under the mucosal surface of the lateral floor of the mouth, along the lingual surface of the mandible, located anterior to the submandibular gland. The gland has several secretory ducts that empty to the oral cavity through the oral mucosa, although on occasion they can become confluent and empty as a single duct into the Wharton’s duct. Histologically, the sublingual glands are composed predominantly of mucous glands (Fig. 14.2C).
Epidemiology
The incidence of salivary gland tumor in the US is in the range of 1–3 per 100 000 population.1 This represents less than 3% of all tumors of the body, and the largest bulk of these salivary gland tumors are found in the parotid gland. In fact, the proportion of tumors of the parotid gland compared to the submandibular gland and sublingual gland are 100 : 10 : 1.2
While the vast majority of salivary gland tumors are found in the parotid gland, it is important to note that 80% are benign.3 On the other hand, 40% of tumors of the submandibular gland and 60% of tumors of the sublingual gland are found to be malignant4 (Table 14.1). While tumors of the minor salivary glands are uncommon, 60–80% are malignant.5–7 No etiological factors have been found for the development of tumors of the salivary glands. However, it has been shown that there is a high incidence of malignant tumors of the salivary glands among patients who had been exposed to previous radiation.8
Table 14.1 Tumors of the salivary glands
| Benign | Malignant | |
|---|---|---|
| Parotid | 80% | 20% |
| Submaxillary | 60% | 40% |
| Sublingual | 40% | 60% |
| Minor salivary glands | 20% | 80% |
An association between radiation exposure and salivary gland tumors was first identified in survivors of the atomic bomb in Hiroshima, Japan. One investigation selected 66 patients for a study to evaluate whether there was an increased incidence of malignant salivary gland tumors among individuals with exposure to radiation. Among the atomic bomb survivors, 52.8% (19/36) had malignant salivary gland tumors, while only 16.7% (5/30) of the nonexposed population developed malignant tumors.9 A further analysis of this data determined that the risk was highest, but not limited to, mucoepidermoid tumors.10 These reports have been followed by a more recent follow-up study of survivors evaluating dose of radiation exposure from the atomic bomb. In this study, there was an increase only in mucoepidermoid and Warthin’s tumors among individuals with an increased radiation dose (estimated radiation dose according to the dosimetry system; high exposure relative risk 9.3 for mucoepidermoid cancer (11 cases) and relative risk 4.1 for Warthin’s tumor (12 cases)).11
On the other hand, low-dose radiation has also been shown to be associated with subsequent malignant transformation of salivary gland tumors. A retrospective analysis of Israeli children who were treated with low-dose radiation for tinea capitis at the time of their immigration between 1949 and 1960 revealed an increase in parotid tumors (4/1000 in irradiated group versus 0/1000 in population control).12 In another study, a population of patients receiving X-ray therapy for acne in Los Angeles revealed an increased incidence of malignant parotid tumors. Those patients who had received more than 15 such treatments had an 8.0 relative risk, and it is estimated that 28% of malignant tumors that occurred in Los Angeles from 1976 to 1984 were attributable to radiation.13 All these reports suggest that there is a lag phase between the radiation exposure and the malignant transformation of the salivary and endocrine glands. In order to explore this further, a study of patients who had received head and neck irradiation to the tonsillar area and the nasopharynx demonstrated the interval between radiation exposure and tumor diagnosis was from 7 to 32 years.14 For these reasons, it is essential that patients who have received radiation should be continually followed for the potential of developing subsequent salivary gland tumors.
Diagnosis/patient presentation
Fine-needle aspiration
Germane to this discussion is the accuracy of FNA in the context of salivary gland tumors. The sensitivity and specificity of FNA for salivary gland tumors reported in the literature range from a high of 99% sensitivity and 100% specificity, as reported by Bhatia,15 to a low of 90% sensitivity and 75% specificity, as reported by Cohen and colleagues.16 In general, however, recent series document a trend towards a higher degree of sensitivity and specificity.17,18 A higher degree of accuracy has been reported for benign tumors.19–21
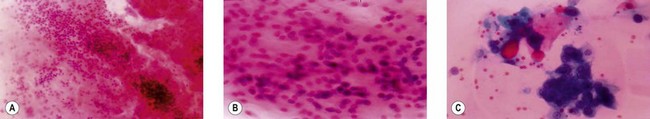
The common diagnostic pitfalls involve distinction between benign oncocytic tumors and acinic cell carcinomas, pleomorphic adenomas and adenoid cystic carcinomas, high-grade mucoepidermoid carcinoma and metastatic squamous cell carcinoma, and finally low-grade mucoepidermoid carcinoma and Warthin’s tumor (Fig. 14.5). Mucoepidermoid carcinomas appear to be the most difficult to diagnose by FNA.22 In an effort to improve diagnostic accuracy of aspirates, special immunohistochemical stains such as glial fibrillary acid protein for pleomorphic adenomas23 and silver staining of nucleolar organizer region have been used.24
FNA is essentially free of complications. Hemorrhage and necrosis of the tumor following aspiration of a lymphoma21 and a Warthin’s tumor25 have been reported, but such occurrences are exceedingly rare. The concerns regarding needle track seeding have been specifically addressed by several authors,21,26,27 who have found no cause for alarm because of its exceedingly low occurrence.
The technique of FNA we use is still essentially the same as that reported previously by us in 1981.28 A 21-gauge needle is affixed to a 10-cc controlled syringe, retaining no air in the syringe barrel, and applying negative pressure by withdrawing the plunger. A minimum of two passes is made into the tumor. The needle is withdrawn and removed from the syringe, air is drawn into the cylinder, the needle is reapplied to the syringe, and a tiny droplet of specimen is applied to the glass slide to prepare a slide smear. The slides are placed immediately into a jar of absolute or 95% alcohol. Next, alcohol is withdrawn through the needle into the syringe and flushed a few times into another jar of absolute alcohol to prepare a cell block.
Imaging modalities
Computed tomography
The ready availability and the cost of this imaging modality account for the popularity of CT scans if additional imaging studies are required by the clinician. While the ultimate choice of a CT scan or MRI may be dictated by nonclinical factors, the consensus is that CT scans are better in a patient with a history of inflammatory disorder while MRI is the modality of choice for palpable masses.29,30 Specific clinical scenarios, as discussed below, may mandate the use of CT scans.
Magnetic resonance imaging
The distinction between benign and malignant tumors on MRI is predicated upon the difference in water content between the two types of tumor. The distinction however is not absolute. Som and Curtin30 point out that high-grade malignancies tend to have low to intermediate signal intensities on all imaging sequences. Well-differentiated tumors, on the other hand, which include benign tumors and low-grade malignancies, tend to have a low T1 and high T2 signal intensity (Table 14.2). This is explained by the fact that low-grade malignancies are generally well differentiated enough to produce secretory products, which therefore yield a higher net water content, reflected by a high T2 signal.
Table 14.2 Distinction between benign and malignant tumors by magnetic resonance imaging
| T1 | T2 | |
|---|---|---|
| Lymphoma | Low | High |
| Malignancy (high-grade) | Low | Low |
| Lipoma | Fat signal | Fat signal |
| Warthin’s tumor | Intermediate | High |
| Pleomorphic adenomas | Low to intermediate | High |
| Hemangioma* | Intermediate | High |
* Signal voids representing large-vessel phleboliths are characteristic. Venous malformations are best diagnosed by magnetic resonance imaging; true arteriovenous fistulae require an arteriogram for diagnosis.
Contrast agents were developed to improve image resolution on MRI; among these, gadolinium-containing compounds are the most widely used. Gadolinium chelates were developed because of the high relaxivity of the gadolinium ion coupled with the relatively low toxicity of the complex with chelation of the metal ion.31 Gadolinium therefore enhances lesion identification and characterization. Some authors have suggested that the routine use of gadolinium in MR studies of the salivary glands improves the quality of data obtained. The consensus, however, is that this adjunct is not necessary for the majority of cases.30
Kramer and Mafee32 note that the intensity of the signal of the parotid gland is slightly less than that of subcutaneous fat, and greater relative to muscle on T1-weighted, proton density-weighted, and T2-weighted images. Additionally the submandibular gland has a slightly lower signal intensity than the sublingual gland on proton density-weighted and T2-weighted sequences. This enables the distinction of the deep segment of the submandibular gland from the sublingual gland. These authors recommend conventional transverse T1-weighted and fast spin echo or short T1 inversion recovery T2-weighted techniques without gadolinium for tumors. Intravenous injection of paramagnetic contrast material, however, is extremely useful in evaluating perineural spread and cervical node involvement. MRI is particularly advantageous in delineating the margins of a lesion, thereby enabling clinicians to distinguish between multiple masses and lobulated solitary lesions. These differences in signal intensity as seen by MRI help to differentiate between the various neoplasms involving the salivary gland (Table 14.2).
Sialography
Sialography is an invasive procedure which involves identification of the ductal opening of the gland to be studied, cannulation, injection of contrast material, and obtaining views in different planes. Sialograms or contrast delineation of the ductal structure of a salivary gland have very little application today in the face of near-perfect images produced by MRIs and CT scans.33 Sialograms are useful in demonstrating ductal calculi and ductal disruptions secondary to trauma. Sialograms are said to be more sensitive than MRI in the diagnosis of Sjögren’s syndrome in the early stages of the disease.30 The sialographic appearance of Sjögren’s syndrome is characterized by a uniformly distributed, punctate accumulation of contrast material throughout the gland, graphically described as a “leafless fruit-laden tree.”30
Classification of tumors
The simplest way to classify salivary gland tumors is by their histologic cell types (epithelial or nonepithelial) and by their behavior patterns (benign or malignant) (Table 14.3).
Table 14.3 Salivary gland tumors
| Primary tumors | |
| Benign | |
| Epithelial | Pleomorphic adenoma (benign mixed tumor) |
| Monomorphic adenoma | |
| Papillary cystadenoma lymphomatosum (Warthin’s tumor) | |
| Oncocytoma | |
| Nonepithelial | Hemangioma |
| Malignant | |
| Epithelial | Mucoepidermoid carcinoma |
| Adenoid cystic carcinoma (cylindroma) | |
| Acinic cell carcinoma | |
| Malignant mixed tumor | |
| Squamous cell carcinoma | |
| Adenocarcinoma | |
| Oncocytic carcinoma | |
| Nonepithelial | Lymphoma |
| Metastatic tumors to salivary glands | |
| Melanoma | |
| Thyroid | |
| Kidney | |
| Breast | |
| Lung | |
| Colon | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree