Right Lobe Liver Transplantation in Adults
James J. Pomposelli
DEFINITION
Right lobe liver transplantation is defined as the use of a donor graft comprising segments 5 to 8 with or without inclusion of the middle hepatic vein for transplantation into a recipient with end-stage liver disease, certain metabolic conditions, or unresectable hepatocellular carcinoma (HCC).
PATIENT HISTORY AND PHYSICAL EXAM
Potential recipients should undergo multidisciplinary evaluation at an accredited transplant center and include medical, anatomic, and psychosocial studies. In cases of living donation, the recipient evaluation must be independent from the donor evaluation to avoid conflict of interests.
Medically acceptable indications for liver transplant vary by country and governing and regulatory agencies. Recipients with a history of drug and alcohol abuse should demonstrate abstinence and be enrolled in a treatment program to maintain sobriety. Expected outcome after live donor liver transplantation should be similar to or exceed outcomes using deceased donors. Because donor safety and transplant use is paramount, performing live donor liver transplantation in recipients with poor predicted survival should be avoided.
Anatomic evaluation should evaluate body size, vascular patency, and the presence or absence of HCC. Adequate graft volume for recipients varies depending on the underlying disease severity but should provide the recipient greater than 30% standard liver volume (SLV) or graft weight-to-body weight ratio (GW/BW) of approximately greater than 0.8.1,2 Extremely small grafts, as small as a GW/BW of 0.4, have been successfully used. Patients with well-compensated cirrhosis (low Model for End-Stage Liver Disease [MELD] score) and unresectable HCC can tolerate smaller sized grafts. Recipients with more advanced liver disease may require larger grafts.
Morbidly obese recipients are not ideal candidates for right lobe grafts due to limits of graft size and GW/BW.
IMAGING AND OTHER DIAGNOSTIC STUDIES
Donors and recipient pairs should have compatible blood type. Although not common with deceased donors due to time constraints with procurement and placement of organs, human leukocyte antigen (HLA) typing and crossmatching can be helpful in avoiding acute cellular rejection and autoimmune reactions.
For immunologically incompatible donor recipient pairs, desensitization protocols may be performed.
Anatomic evaluation should be performed by either computed tomography (CT) or magnetic resonance imaging (MRI) scanning. Arterial and venous phase imaging allow for identification of arterial anomalies and venous patency. Current established imaging protocols can determine the diagnosis of HCC without biopsy.3
SURGICAL MANAGEMENT
Preoperative Planning
Informed consent process should include review of the recipients’ most recent evaluation data by the primary surgeon to determine suitability for transplant. Risks, benefits, center-specific outcome data, and need for lifelong immunosuppression should be discussed.
Patients are prepared by anesthesia personnel with large-bore intravenous access, invasive arterial pressure monitoring, and may benefit from intraoperative cardiac performance monitoring (i.e., Swan-Ganz catheter or transesophageal echocardiography [TEE]).
Antibiotic prophylaxis should provide broad-spectrum coverage to include skin and biliary flora (i.e., Ceftriaxone).
Venous thromboembolism (VTE) prophylaxis with sequential compression devices is desirable. Due to underlying coagulopathy, routine use of subcutaneous heparin is not needed.
Medications to reduce portal pressure (e.g., octreotide, vasopressin) may be helpful during recipient hepatectomy to reduce blood loss and after graft reperfusion to reduce hyperperfusion and portal hypertension sequelae in the live donor graft.
TECHNIQUES
RECIPIENT HEPATECTOMY
The type of surgical incision is dictated by surgeon preference but should provide excellent exposure and easy access to the hilum of the liver and vena cava. Bisubcostal “chevron” incision or right subcostal with upper midline vertical extension are most commonly used.
Complete dissection of the hepatic hilum before mobilization of the liver reduces blood loss by delaying the dissection of retroperitoneal varices.
Design the hilar dissection to maximize the length of the hepatic artery and portal vein without compromising the vasculature to the bile duct.
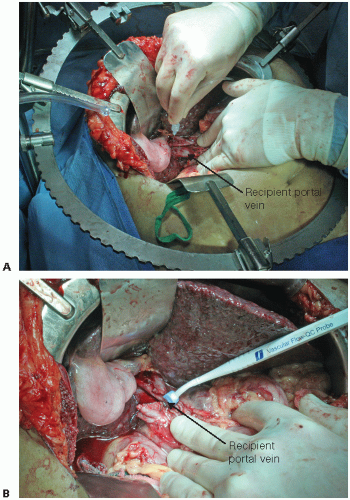
Measure portal pressure and flow before and after graft implantation. This informs the decision to perform inflow modification to prevent hyperperfusion and small for size syndrome (SFSS) (FIG 1).4
Absolute portal pressure above 20 mmHg (unclamped portal pressure) or portal gradient (portal pressure minus central venous pressure [CVP])
greater than 10 mmHg warrants consideration for inflow modification.
After measurements of flow and pressure, divide the hepatic artery.
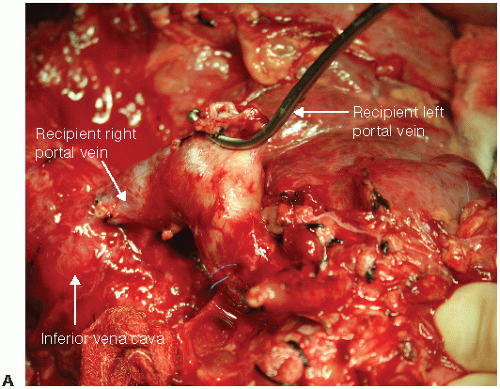
FIG 2 • Techniques for hemiportacaval shunts. Hemiportacaval shunts can be performed using the recipient’s portal vein bifurcation (A), side-to-side using main portal vein (continued)
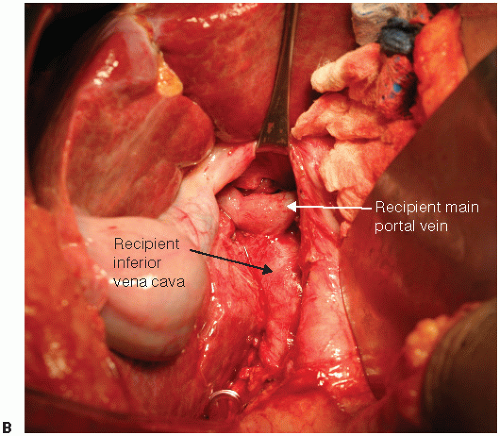
FIG 2 • (continued) (B), or interposition graft using a variety of conduits. A 6-mm PTFE graft can also be used. Type of shunt is dictated by graft placement and portal vein length.
Temporary end-to-side portacaval shunt or venovenous bypass can be performed early after hilar dissection to improve hemodynamics and reduce blood loss. For cases where inflow modification is used, hemiportacaval shunt can be performed with clamping of distal end (FIG 2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree