12 Reconstructive surgery of the mutilated hand
Introduction
Key points
• Thorough debridement is essential in the management of these patients to decrease risk of infection and scarring
• In cases of arterial insufficiency, provisional revascularization via a shunt or temporary vein graft should be considered
• Adequate skeletal stabilization should be performed early and preferentially is done with internal fixation to allow definitive repairs of neurovascular structures and soft-tissue coverage
• Definitive vascular reconstruction is usually delayed until after skeletal stabilization is achieved
• Musculotendinous reconstruction should be done early if possible, to the point of performing early tendon transfers
• Nerve repair is also best performed early, to allow proper alignment of nerves and to avoid having to dissect the nerve out of scar at a secondary procedure
• Proper management of the soft-tissue envelope is essential to allow all of the other structures to be repaired as noted above. Proper flap selection allows early rehabilitation and facilitates secondary surgery when necessary
• Postoperative management revolves around proper planning at the first stage of reconstruction. Every surgery should be performed with an eye to what will need to be done in the future
Debridement
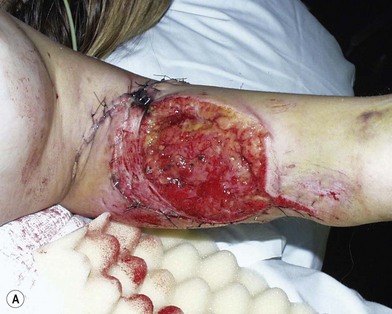
Radical debridement with elimination of all nonviable tissue is the crucial step in the management of these injuries. This is performed under tourniquet control to provide the best visualization of the extent of injury and to prevent iatrogenic injury to intact structures. Once nerves and vessels are identified, the tourniquet may be released to assess the viability of the remaining tissue better (Fig. 12.1).
Provisional revascularization
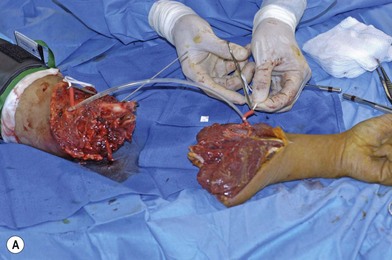
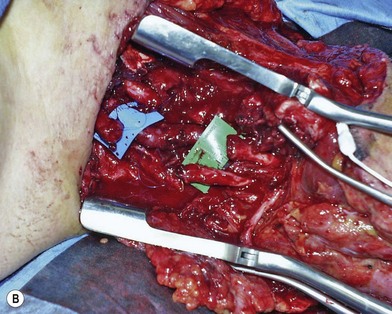
When the extremity is amputated or ischemic and the delay to surgery or anticipated length of time for debridement and definitive skeletal stabilization exceeds 6 hours, provisional revascularization can be done with use of a shunt, such as a Javid, Ishihara, or comparable segment of plastic tubing (Fig. 12.2). While different shunts are available, their use is similar. Whichever shunt is chosen (primarily by what might be available for use by the vascular surgeons), it is generally flushed with heparinized saline and then clamped. The concentration of this is usually 100 units heparin per cc saline. The shunt is then carefully slipped into the ends of the vessels to be shunted (artery to artery). The shunt is either held in place with moist umbilical tapes placed around the vessels and clamped tight with a piece of red rubber catheter (Fig. 12.2), or some shunts come with a special clamp which goes around the artery and holds it flush on the shunt. While generally utilized for only short periods of time (i.e., minutes to a few hours), these types of shunt have recently been utilized to great effect in the conflict in the Middle East for up to 8 hours on major arteries, without systemic heparinization. In this application, however, their use should only be necessary for the time it will take to perform an initial debridement and bony fixation. Another option is rapidly to perform reversed vein grafting between the arterial ends to re-establish arterial inflow. After definitive skeletal stabilization, the length of the vein graft can be adjusted and the anastomosis revised.
Vascular reconstruction
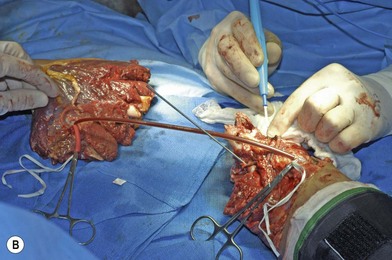
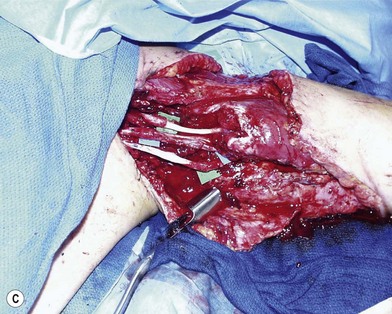
Definitive revascularization is done once skeletal stabilization is completed. Lacerated vessels are resected to healthy-appearing vessel wall both proximally and distally. Contusion along the adventitia suggests injury within the intimal layer as well. A “ribbon sign” (convoluted or tortuous course of the digital vessels) indicates injury to the media layer of the vessel and requires resection of the length of the involved area and reversed vein grafting (Fig. 12.3). Inflow is assessed, and if it is not adequate, the vessel should be resected more proximally until pulsatile flow is achieved.
When the need for coverage and vascular repair present themselves simultaneously, one can consider “flow-through” free flaps both to bridge the arterial defect and to obtain coverage at the same time. The radial forearm free flap is very useful in this regard, as the radial artery offers an excellent choice for a long bypass.1 Due to trauma to the forearm, however, this may not be an appropriate choice of flap. In this case, the anterolateral thigh flap with its long segment of the descending branch of the lateral femoral circumflex can be utilized.2,3 Small venous free flaps with arterialized segments of vein can be utilized to great utility for revascularization of fingers in the face of a soft-tissue loss volarly.4,5
Musculotendinous reconstruction
Functional reconstruction of muscle deficits and tendon injuries should be done immediately whenever possible. Unrecoverable muscle function can be treated with tendon transfer, and it is preferable to perform this as a primary procedure. However, this can also be performed at a later stage of reconstruction. Delayed transfers are generally more difficult because they have to be performed through a scarred tissue bed, and this requires additional surgical procedures and further delays the patient’s rehabilitation and recovery. When no donor tendons are available for transfer, muscle function can be restored with functional free muscle transfer. The gracilis is the most commonly used muscle for functional reconstruction and can actually provide both coverage and restoration of muscle function to the fingers. Again, this can be done either primarily or at a later stage of reconstruction; however primary reconstruction is favored as available nerves and vessels are easier to locate and one is not operating through a scarred bed of tissue. This procedure will be discussed in more detail below and in Chapter 35.
Nerves
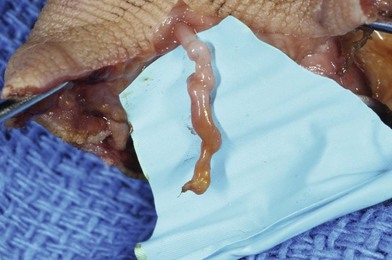
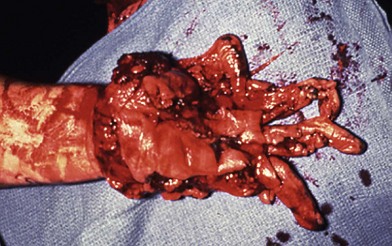
Mobilizing the proximal and distal stumps to achieve primary repair is not recommended because this results in devascularization of large segments of the nerve. Mobilization can be done over a 1–2-cm distance to allow repair, but to avoid repair under tension, nerve grafting is preferable. If a staged repair is planned, nerve ends are tagged with 6-0 polypropylene suture for later identification. Primary nerve grafting is recommended, however, as the orientation is much clearer at the time of injury rather than later and dissecting a nerve out of a scarred bed is technically challenging (Fig. 12.4). Common donor nerves include the sural or saphenous nerves, sensory branch of the radial nerve (if lacerated from the injury), medial or lateral antebrachial cutaneous nerves, and the posterior interosseous nerve. In multiple nerve injuries, primary nerve transfers can be performed.6 These include transfer of the anterior interosseous nerve (distal to the branch to the flexor pollicis longus) to the motor branch of the ulnar nerve and transfer of the sensory branch of the radial nerve to the digital nerves of the thumb and index finger.7 When a nerve defect is greater than 15 cm, an end-to-side neurorrhaphy of the distal stump of the injured nerve to a neighboring intact major nerve can be done, although functional outcomes of this procedure are generally poor.
Basic science/disease process
The term “mangled” is commonly used to describe the hand and upper extremity after major trauma. Gregory et al. used the term “mangled” to describe a severe injury to at least three of the four organ and tissue systems of skin, bone, vessel, and nerve.8 According to the Oxford English Dictionary, to mangle is “to reduce by cutting, tearing or crushing to a more or less unrecognizable condition.” Each of these definitions implies a severe, high-energy injury that involves multiple anatomic structures, usually over an extended topography.
Mangling injuries are produced by high-energy forces. High-power equipment – agricultural (corn picker, grain auger), industrial (punch press, power saw), or household (lawn mower, snow blower) – may cause such an injury (Fig. 12.5). In addition, gunshot wounds, explosives, and motor vehicle accidents (especially with the arm of the patient being outside the car window) account for many cases. The injury may have a combination of sharp, crushing, avulsive, and thermal components. The wound may be severely contaminated, depending on the location and mechanism of injury.
Types of injury
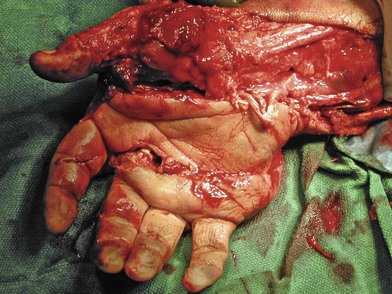
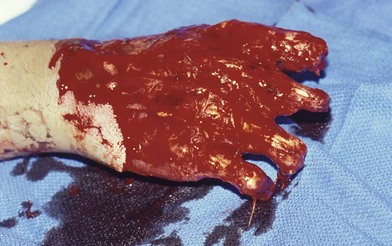
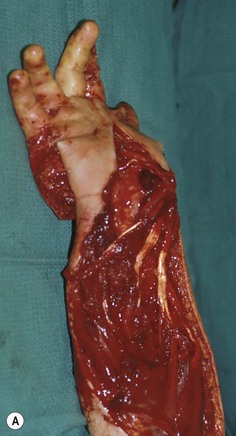
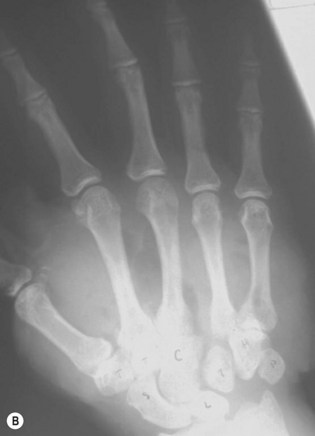
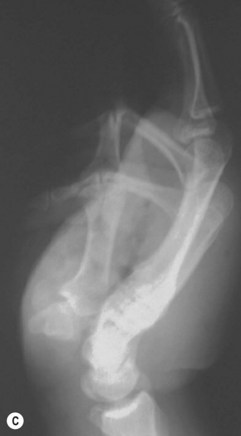
Avulsion injuries primarily involve the soft tissues of the upper extremity. The etiology of these injuries is often due to industrial accidents in roller press machines which both cause bone dislocations in the hand and can lead to loss of the entire soft-tissue envelope to the hand (Fig. 12.6). These injuries predictably lead to severe scarring and often poor function despite the type of reconstruction performed. Early motion is essential for reasonable outcomes in these injuries.
Roller injuries occur when the hand is caught between two rollers whose function is usually to compress sheets of metal. This can result in an array of injuries, including degloving of the skin of the hand, fractures, and potentially crushing of all the tissues of the hand and forearm. These are devastating injuries and call on the entire armamentarium of the surgeon to manage the bony fractures, dislocations, and coverage and functional issues from soft-tissue damage (Fig. 12.7).
Diagnosis/patient presentation
Evaluation of the patient in the emergency room
In the case of limb-threatening ischemia in a mangling injury, evaluation of the status of the vessels is generally performed in the operating room without the delay necessary for an arteriogram. An intraoperative angiogram may aid in determining the level and extent of arterial injuries, however. Radiographs taken in the operating room are usually of better quality than those taken in the emergency department because the extremity can be positioned without causing the patient discomfort. Traction radiographs allow better delineation of the fracture pattern and number of fragments, especially in evaluating intra-articular fractures about the wrist and elbow. Photographic documentation of the injury should be done throughout the course of treatment from initial evaluation to conclusion of treatment.9
Emergency room to operating room – making a plan
Planning for reconstruction
The multitude of factors and the complex interrelations among them make reaching a decision a difficult task, even for experienced surgeons. Specialized scoring systems have been developed on the basis of lower-extremity injuries. These may offer valuable guidelines for evaluating the lower extremity but cannot be applied well to the upper extremity.10 Each case is unique, however, and the final decision should be an individualized one based on assessment of the patient and extremity parameters as well as sound judgment. The patient’s knowledge of the potential risks and benefits of surgery and the possibility of early or later amputation is important.
A third factor which is rarely discussed is the surgeon factor. The skill and experience of the surgeon managing the patient are extremely important in determining the outcome.11 While the experienced surgeon is more likely to have a more favorable outcome, reasonable results can be obtained by others if they follow basic principles and stick to what they are comfortable doing. The surgeon who only occasionally performs microsurgical tissue transfer may better serve the patient by performing a pedicled flap for initial coverage than attempting an esoteric and complex microsurgical procedure. It is always better to perform an initial reconstruction using a technique with which you are familiar rather than attempting to perform a procedure that has a high potential for failure. In any case, it is preferable to refer a patient with a complex extremity injury to a center which routinely deals with these problems rather than attempt reconstruction if one is not familiar with these types of problems.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree