11 Reconstruction of the soft tissues of the back
Synopsis
 Reconstruction of the soft tissues of the back at first may seem to be a daunting task compounded by large wounds, unfamiliar and segmental anatomy, radiation, hardware, and difficulties with postoperative positioning.
Reconstruction of the soft tissues of the back at first may seem to be a daunting task compounded by large wounds, unfamiliar and segmental anatomy, radiation, hardware, and difficulties with postoperative positioning.
 Many of the conditions treated require significant coordination with surgical colleagues.
Many of the conditions treated require significant coordination with surgical colleagues.
 Many of the conditions are unfamiliar to the plastic surgeon and without parallel conditions elsewhere in the body, an example being pseudomeningoceles filled with cerebrospinal fluid (CSF).
Many of the conditions are unfamiliar to the plastic surgeon and without parallel conditions elsewhere in the body, an example being pseudomeningoceles filled with cerebrospinal fluid (CSF).
 The goal of this chapter is to provide the reader with real-life solutions to difficult problems involving back wounds.
The goal of this chapter is to provide the reader with real-life solutions to difficult problems involving back wounds.
Patient treatment
Local wound care
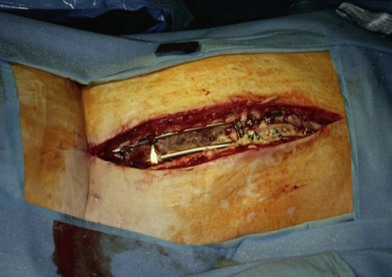
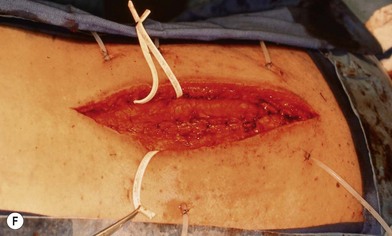
Much depends on the patient’s home situation. Dressings can be simple wet-to-wet saline dressings, with twice-a-day showers to cleanse the surface of the wound. Subatmospheric-pressure dressings can be employed, but the tubing is sometimes difficult to place under back-bracing devices (Figs 11.1–11.3). After a wound has granulated, if it is painful, then delayed primary closure or skin grafts can be performed if the wound is sizeable. However, the risks of these secondary closure procedures often outweigh the benefits. The subcutaneous tissue stiffens during the time of obtaining local wound control, and it is difficult to re-elevate and close without a fairly sizeable procedure.
Operative debridement
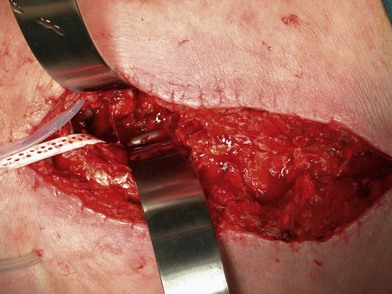
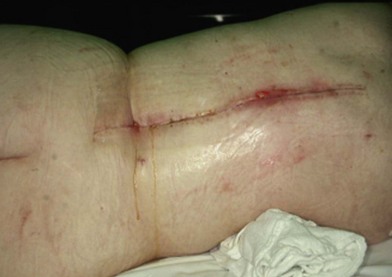
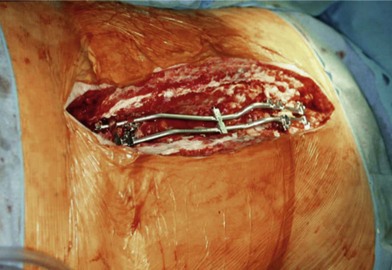
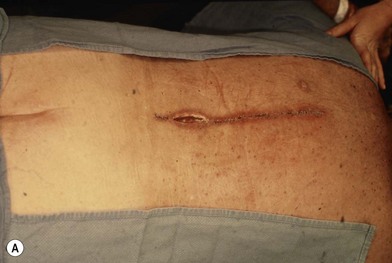
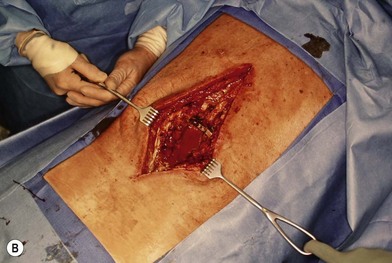
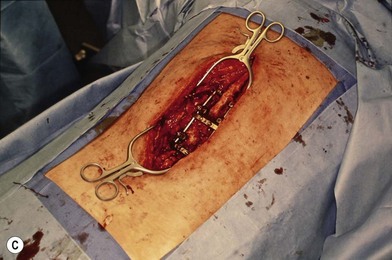
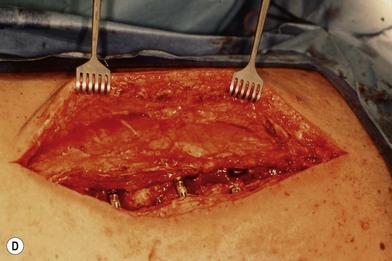
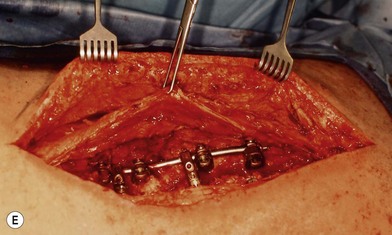
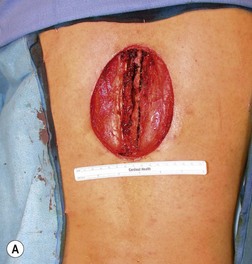
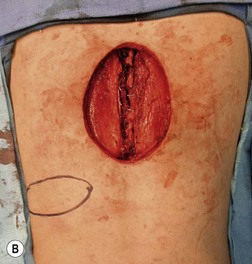
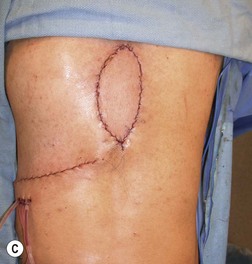
Wound “shape” is an important concept for reconstruction of the soft tissues of the back. The back has lordotic and kyphotic regions in the normal condition, and these contours can be dramatically changed with pathology. This serves to make postoperative positioning difficult when the surgical incision is also the most prominent portion of the back. The depth of the procedure performed by the spine surgeons is also important. A patient with a laminectomy by definition has a deeper wound than when the spinous processes are intact. Spinal hardware serves to create a local prominence in contradistinction to the depth of the surgical dissection. A prime reason for persistent fluid collections is the three-dimensional space between a laminectomy and the adjacent vertically oriented bars. Crosslinks between the bars further prevent soft tissue from collapsing into the space immediately over the midline. Finally, laterally placed hardware can be poorly covered by soft tissue and may be the originators of pressure sores. This is especially common when hardware inserts into the posterior superior iliac spines for pelvic fusions. Therefore, in the treatment of wounds of the spine, the three-dimensional shape should be evaluated and converted as much as possible to a two-dimensional wound. Prominent hardware should be exchanged for something with a lower profile (Figs 11.4–11.7). Patients with incomplete corrections of the spine deformities should be revised to recreate better the natural contours. The deeper the hole, the more a flap should be “dropped into” the defect, rather than tissue simply slid towards the midline. This requires a more redundant soft-tissue flap such as an omentum to fill the defect appropriately. Teamwork between the spine and soft-tissue surgeons is often necessary to treat problems of wound shape and contour appropriately.
Fig. 11.7 Healed wound in this patient after optimizing both the hardware architecture and the soft tissues.
Flap closure
Principles
Central questions to be answered include the presence or absence of a fusion, and the vertebral levels involved.1 When an instrumented fusion has been performed, then the erector spinae musculature function is no longer necessary, and the muscles are completely expendable in terms of a reconstruction. The fusion rods prevent postoperative motion, and so when the erector spinae muscles are reapproximated in the midline, they tend to stay there. Spine patients without fusions still require the function of the erector spinae musculature when healing is completed for flexion and extension of the spine. These patients do better with flaps that are “dropped into the hole,” rather than with erector spinae muscles that are closed side to side and that would dehisce with back flexion.
Possible flap choices for spine closure
Erector spinae muscle flaps
The erector spinae muscles, also called the paraspinous muscles,2 are expendable after a previous spine fusion and no longer are functional for spine extension and flexion. The dissection of the muscles proceeds in stepwise fashion in order to move the muscles towards this midline. This flap is appropriate from the high cervical area to the low lumbar area, but it will not adequately cover an occipitospinal fusion, and nor will it be sufficient for lumbosacral soft-tissue coverage. One must be careful in its use when a lateral approach to the spine has been made, because the muscle can be transected for access.
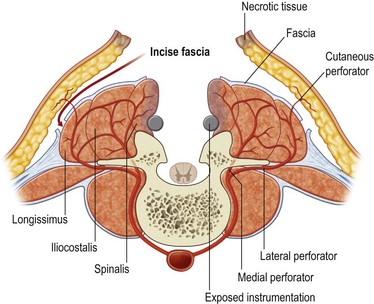
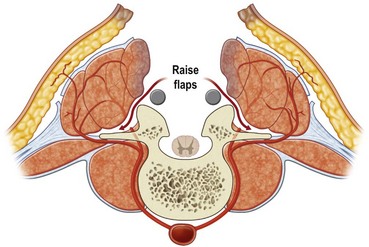
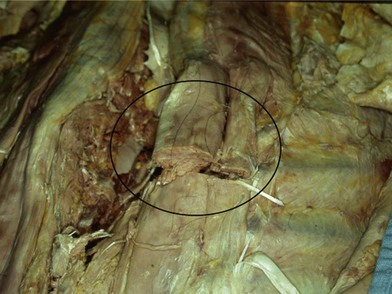
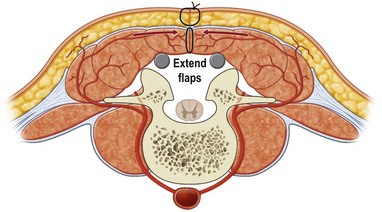
First, skin flaps are elevated superficial to the thoracolumbar fascia (Fig. 11.8). With a retractor putting tension on the soft tissues, with cautery the granulation tissue is entered, and with finger dissection a blunt plane is elevated. The latissimus muscle and trapezius muscle should stay attached to the skin. The dissection is easiest inferior in the lumbar area where the muscle is round and large, and most confusing superiorly where the muscle is thinnest and becomes attached to the undersurface of the trapezius. The erector spinae muscles have a convex shape, and there is a rounded aspect of the muscle that then descends laterally towards the more lateral neck, thoracic, and lumbar areas. It is in this groove that segmental blood vessels enter the lateral and deep aspects of the longissimus and iliocostalis muscles. Continuation of these blood vessels continues more superficially to the skin and to the latissimus muscle in the thoracolumbar area. While the surgeon is elevating the skin flaps, the perforators and dorsal sensory nerves going up to the skin often can be identified and preserved to maintain skin vascularity. To lessen the chance of marginal skin perfusion and to limit a future potential seroma cavity, undermining should not be overdone.
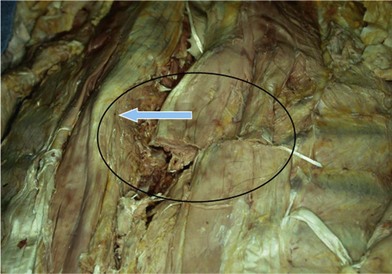
The thoracolumbar fascia is incised much like the development of any standard bipedicled flap in order to mobilize the erector spinae muscle and/or overlying skin to the midline. When the original dissection is superficial to the fascia and the fascia remains attached to the muscle, this facilitates the later closure, and the fascial release is critical to the movement of the muscle. Occasionally the fascia remains attached to the skin during the initial elevation. In this case, muscle mobilization is facile, but the skin is more difficult to close in the midline. Therefore, an incision of the fascia along its length allows the skin to move medially and to provide the excess for debridement and reclosure. The dissection up until this point is rather easy and bloodless, but only moves the muscle an estimated 30% of its potential. Complete mobilization of the muscle requires a dissection along the deep aspect of the erector spinae (Fig. 11.9). Again with tension applied on to the tissue with retractors, cautery dissection of the deep and medial attachments of the erector spinae will mobilize the muscle and detach it from the lateral aspects of the transverse processes of the spine. It will by necessity divide the medial row of blood vessels entering the paraspinous muscles, but the prior dissection to identify the lateral vessels entering the muscle will suffice to preserve vascularity. One technique is to have the fingers of the nondominant hand in the lateral groove at the site of the lateral perforators while bluntly elevating the medial aspect of the muscle with the thumb and index finger. The medial muscle elevation is a powerful means to allow the paraspinous muscles to “unfold” like an accordion to be advanced toward the midline (Figs 11.10 and 11.11). The muscle changes shape from being a round muscle mass to being more elliptical. Advancement of the muscle is a process much different from simply releasing the thoracolumbar fascia and “rolling” the dorsal aspect of the muscle toward the midline.
With the skin and muscle flaps so created, it is now quite easy to distinguish stiff and inflamed tissue from more normal soft tissue. The medial aspect of the skin, subcutaneous tissue, and paraspinous muscles is sharply debrided. The muscles are brought together in the midline (Fig. 11.12), and any extra tissue can be imbricated to help fold the soft tissue into crevices between vertically oriented hardware bars (Fig. 11.13). Drains are left both deep and superficial to the erector spinae closure, and are left in until the drainage is minimal. When the erector spinae muscle closure is of good quality, there is no need for a second overlying muscle flap using the trapezius or the latissimus muscles.
Latissimus muscle or myocutaneous flap
The latissimus muscle is a well-known and understood flap.3 The donor site is minimal. Based on the thoracodorsal pedicle, the muscle can be moved superiorly up to the level of the top of the scapula. Based on its minor perforators that also supply the paraspinous muscles, the latissimus can reach the lower lumbar area. An advantage to the latissimus is being able to be “dropped in” to a hole, and so it is useful for patients who have not been fused, or for more lateral defects. The muscle can reliably carry a skin paddle, and this often helps in the inset of the flap. Caution should be taken in patients who have had thoracotomy incisions, as the muscle is often divided.
The latissimus muscle is a good but still second-line flap for the coverage of midline back wounds. In comparison to the paraspinous flaps, the latissimus flap can only cover a spine wound 10–12 cm in length, while the erector spinae flaps can cover practically the entire length of the spine. The dissection required to elevate the latissimus muscle takes more time and effort than does the erector spinae. While low, there is a donor defect from the loss of the latissimus muscle, especially in patients who may need assistive devices to ambulate. The latissimus flap is a good choice for nonfused wounds of the back (Fig. 11.14), or when the erector spinae muscles have been radiated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree