div class=”ChapterContextInformation”>
33. Robot-Assisted Reconstruction of the Lower Urinary Tract
Keywords
Reconstructive surgeryRobot-assisted surgeryRobotic surgeryUrologyUrethroplasty33.1 Introduction
Robotic-assisted approaches to posterior urethral stenosis build upon principles of traditional open and endoscopic management. These foundational modalities evolved from experiences with a diverse array of pathologies, each with unique anatomic considerations.
Understanding the role and limitations of these surgical techniques is a critical prelude to discussion of robot-assisted reconstruction. We briefly review the techniques historically utilized for lower urinary tract stricture disease separated by etiology, as the surgical management and approach to the posterior urethra and bladder neck may differ markedly [1, 2].
33.1.1 Radiation-induced Bulbomembranous Urethral Stenosis
Reported rates of urethral stricture following pelvic radiation therapy vary by modality, with a cumulative risk of 1.8–4% after brachytherapy (BT) alone, 1–2% following external beam radiotherapy (EBRT), 5.2–11% after combined EBRT+BT, and nearly 32% with high-dose BT [3, 4]. Clinically significant stenosis may manifest many years after the initial insult [5]. Depending on the proximity and dose of radiation delivered to the periapical urethra, the bladder neck may become more fibrotic and consequently less able to coapt or relax [6]. Poor vascularity of radiated tissues precludes appropriate healing, and radionecrosis of the prostate is common, hampering the reliability of anastomotic sutures [7]. Definitive repair is especially challenging when the stenosis spans the membranous urethra, due to difficulty in accessing this region. In the setting of prior radiation, the likelihood of recurrent stenosis after dilation or direct-vision internal urethrotomy (DVIU) approaches 50%, and repeated endoscopic manipulation may worsen fibrosis and delay definitive management [7, 8].
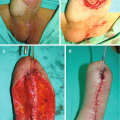
Consequently, consideration for early open surgical repair of bulbomembranous radiation-induced stenosis (RIS) has been advocated. The perineal approach , as described by Hofer et al., involves transection through the stricture, circumferential mobilization of the distal urethra, and aggressive resection of all scarred or necrotic tissue from the proximal urethral stump [5]. If prostatic radionecrosis is encountered, removal of this diseased tissue with a rongeur is performed, including debridement of any involved periosteum of the pubic symphysis. Following clearance of all scar tissue, primary anastomosis is performed in a tension-free manner. Success rates have been reported as 70–85% at long-term follow-up [7]. De novo stress urinary incontinence (SUI) occurs in 33–43% of patients postoperatively, for which staged placement of an artificial urinary sphincter (AUS) is offered [9]. Recurrences were managed with endoscopic balloon dilation or transurethral incision. Critically, these outcomes are derived largely from cohorts of patients with strictures measuring 2 cm or shorter, most of whom had undergone multiple prior endoscopic interventions.
For longer RIS or those with more proximal involvement, adjunctive mobilization techniques including partial pubectomy, partial prostatectomy, or corporal rerouting may be employed. Chung et al. evaluated the incidence of SUI in cohorts of patients undergoing posterior urethroplasty for stricture induced either by radiation or pelvic fracture urethral injury (PFUI) [6]. Most patients with RIS who developed de novo SUI had prostatic urethral involvement (75%), whereas SUI after urethroplasty for PFUI was significantly less common (3%). Adjunctive mobilization measures were undertaken in a majority of RIS cases, whereas these techniques were uncommonly employed in the approach to PFUI. Altogether, these findings imply that the more proximal dissection risks damage to the external sphincter resulting in a greater likelihood of SUI, especially in patients with RIS where the bladder neck may already be damaged. Notably, longer RIS have been associated with greater rates of postoperative SUI, also possibly due to a need for more extensive urethral mobilization [5].
Longer or more proximal strictures may require additional strategies including an abdominoperineal approach , or consideration of flap or graft urethroplasty. The open abdominoperineal anastomosis couples the perineal technique described above with mobilization of the bladder via a midline abdominal incision. An inferior pubectomy with removal of a 2–3 cm fragment is often required to expose the site of the anastomosis [7]. Buccal mucosal graft urethroplasty has been reported to carry a 71–83% success rate but should be accompanied by a vascularized graft bed such as a gracilis flap, because an irradiated bed may not have sufficient vascularity to support a graft [10, 11].
Surgical management of RIS may portend higher risk for complications of subsequent AUS placement. An analysis by McKibben et al. demonstrated that prior urethroplasty and history of pelvic radiation were independently associated with AUS cuff erosion [12]. These complications could result from impaired perfusion of an ischemic segment of urethra attributable to effects of radiation, devascularization from mobilization during urethroplasty or both [13]. Bulbar-artery sparing urethroplasty has been proposed as a method to obviate this potential complication [14], but may be difficult to undertake in the setting of RIS where natural tissue planes can be obliterated and excision of scar tissue is critical.
Despite refinements in open surgical technique, perineal or abdominoperineal approaches to RIS carry a cumulative risk of recurrence of 30% [5]. A significant proportion of patients experience de novo SUI postoperatively, especially with more proximal or longer RIS. Radiation is furthermore associated with an increased risk of AUS cuff erosion, leading to additional morbidity and need for subsequent reconstruction. Conceivably, these adverse outcomes arise due to the aggressive proximal urethral dissection required for adequate open excision of scar and exposure for anastomosis, possibly resulting in damage to the external sphincter and disruption to the bulbar arteries. Techniques involving less urethral mobilization could thus theoretically result in lower rates of de novo SUI or AUS cuff erosion.
33.1.2 Vesicourethral Anastomotic Stenosis
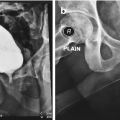
The risks of vesicourethral anastomotic stenosis (VUAS) following radical prostatectomy have been reported at 1–8%, presenting six months postoperatively [2]. In the era of robotic assisted laparoscopic prostatectomy (RALP) the risk has been reduced to less than 3%, presumably due to improved creation of a watertight vesicourethral anastomosis with appropriate mucosal eversion. Owing to the volume of prostatectomy performed worldwide it is likely that VUAS will remain an ongoing reconstructive dilemma [15]. While initial endoscopic management may be appropriate, 42% of patients ultimately require repeat intervention, with markedly reduced success rates for subsequent endoscopic procedures [16]. Specifically, dilation by itself performs poorly, with an overall recurrence-free rate of 13%, while transurethral cold knife incision, hot knife incision, and laser incision have recurrence rates of 25–55% [17]. The median time to recurrence was six months. Intralesional injections have been explored with mixed performance: mitomycin in particular has been associated with no significant change in recurrence rates [18].
Continence outcomes of endoscopic management are difficult to assess given varying degrees of post-prostatectomy incontinence prior to intervention, and lack of uniform reporting of long-term follow-up. Nevertheless, reports of transurethral incision for nonobliterative, short VUAS describe a rate of de novo SUI of 33–50% [19, 20]. Incision was more likely to fail where there was a concomitant history of pelvic radiation, and synchronous membranous urethral strictures were present in a majority of these patients. Staged placement of AUS was required for 92% of patients in a recent series [20].
Nearly one third of patients will have VUAS refractory to three or more attempts at transurethral management [16]. For such patients, or those with long (>2 cm) VUAS, open perineal, abdominal or abdominoperineal approaches have been utilized. Analogous to patients with RIS, goals of these maneuvers include adequate excision of diseased tissue, exposure and mobilization of the distal urethral stump and bladder neck, and tension-free anastomosis. Multiple techniques have been proposed, including abdominoperineal excision, bladder neck reconstruction, and primary excision of the bladder neck with graft urethroplasty [2, 21]. In undertaking such procedures, the patient must be counseled on their extensive nature, length and potentially morbid nature, and the fact that incontinence is highly likely to occur or worsen postoperatively [22]. Risk of rectal, bladder and ureteral injury must be communicated. Prolonged operative time in exaggerated lithotomy position may additionally carry risks for neuropraxia [21]. Nikolavsky et al. report a contemporary series of open VUAS correction for patients with median stenosis length of 2.5 cm [23]. Inferior pubectomy was undertaken in eight of 12 patients due to the need for adequate exposure and visualization, with a mean operative time of 347 minutes and estimated blood loss of 400 cc. While 92% of patients had a patent bladder neck with long-term follow-up, half ultimately required intervention for de novo SUI.
The intraoperative difficulty, perioperative morbidity as well as postoperative incontinence following open repair of VUAS have been noted similarly by other investigators of comparable populations [24, 25]. Nevertheless, the putative advantage of avoiding perineal dissection in this population is that the bulbar urethra is preserved for future AUS if warranted. For long or recurrent VUAS, avoiding trans-sphincteric mobilization of the urethra may be desirable [26, 27]. Endoscopic approaches, while technically more facile, may carry a significant risk for recurrence and may impact continence although to a lesser degree than open correction. Technical advances in definitive repair for recurrent VUAS would ideally decrease operative time and perioperative risk while improving continence outcomes as feasible.
33.1.3 Bladder Neck Contracture
Owing to differences in etiology, epidemiology, length and relation to the external sphincter, bladder neck contracture (BNC) secondary to transurethral resection of prostate (TURP) or laser prostatectomy is discussed separately from VUAS [1]. The cumulative incidence of BNC post TURP is thought to be 1–12%. A single endoscopic intervention may result in a 58–73% durable recurrence-free rate [28]. However, patients who have recurrent BNC after multiple endoscopic interventions may be considered for the same gamut of open reconstructive procedures as with VUAS. In this patient population, open Y-V plasty reconstruction of the bladder neck, described first by Young in 1953 [29], has been described with varying results: the required bladder mobilization and tension on the anastomosis resulted in impaired vascularity and consequent anastomotic breakdown [30]. A modification of this technique, utilizing a T-shaped rather than Y-shaped bladder flap, has been proposed, with the aim of facilitating a tension-free anastomosis; preliminary patency and continence outcomes appear promising [31].
33.1.4 Advantages Offered by Robotic Approaches
The incremental modifications of open surgical techniques form the bedrock of the application of robotics in addressing RIS, PFUI, VUAS and BNC. Improved precision and expanded degrees of freedom while operating in the pelvis, for example, may obviate inferior pubectomy during correction of VUAS [32, 33]. Enhanced visualization may similarly decrease operative time and periprocedural bleeding, with potentially decreased length of hospital stay [34]. Importantly, these technical advances may result in further gains with respect to preservation of continence: by eliminating or reducing the need for trans-sphincteric or bulbar urethral mobilization, damage to the external sphincter or transection of the bulbar arteries may be avoided relative to open approaches to VUAS, RIS or BNC, potentially allowing for improvement in postoperative SUI and reduction in AUS complications.
33.2 Evaluation and Surgical Indications
Indications for robotic posterior urethral and bladder neck reconstruction are identical to those for open abdominal or perineal repair. A robotic approach may be performed as means of primary repair, or after failed prior attempts at open or perineal reconstruction. Patients who have failed endoscopic management of vesicourethral anastomotic stricture or bladder neck contracture following transurethral procedures are candidates for robotic assisted laparoscopic reconstruction using a Y-V plasty approach. Individuals with PFUI or RIS may benefit from a robotic approach to posterior urethroplasty. Likewise, prostatic urethral stenosis can be managed with robotic assisted prostatectomy.
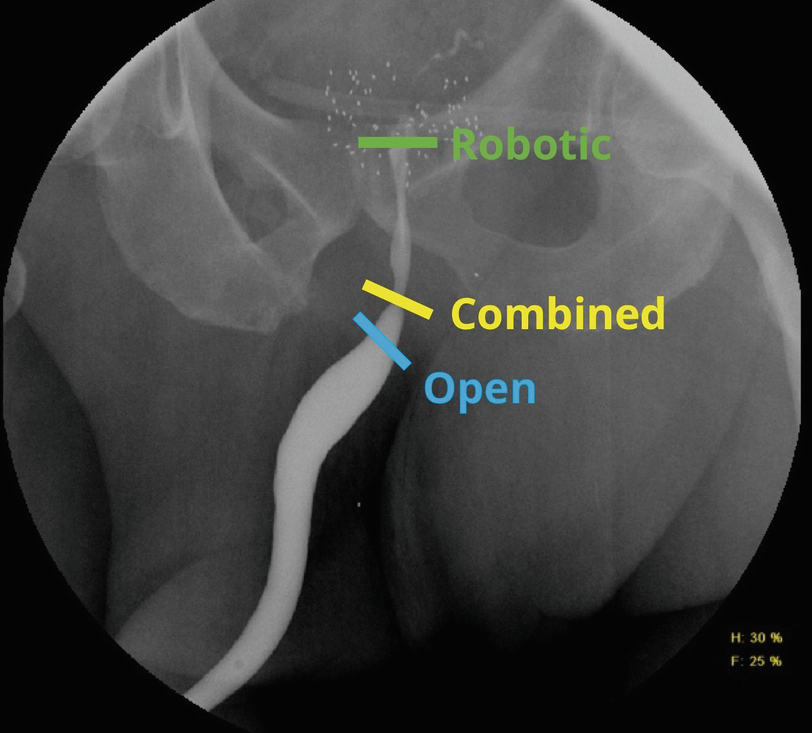
Location of the stenosis or stricture relative to the external sphincter determines the surgical approach. Bladder neck contractures, vesicourethral anastomotic and prostatic urethral stenoses, proximal to the membranous urethra, are approached robotically. Transsphincteric stenoses and strictures may require a combined robotic abdominoperineal approach. Strictures distal to the membranous urethra are best managed with a traditional perineal approach
Patients with urinary retention should undergo suprapubic catheterization in advance of their definitive surgery, to allow for urethral rest for approximately four weeks and subsequent evaluation . Additional work-up may include retrograde urethrogram and voiding cystourethrogram, or sonourethrogram for those who have anterior urethral stricture disease. Concomitant distal urethral strictures should be addressed prior to posterior urethroplasty.
If a patient’s history raises suspicion for compromised bladder capacity or function, urodynamics may be useful in determining which individuals are poor candidates for a robotic approach. In patients with small capacity (100 ml or less), hypocontractile and acontractile bladders, we recommend chronic catheterization or urinary diversion rather than robotic reconstruction. Radiation cystitis that is refractory to hyperbaric oxygen therapy and intravesical therapy is another contraindication to reconstruction.
33.3 Preoperative Preparation
Preoperative urinalysis and culture must be obtained to allow for appropriate preoperative antimicrobial treatment. Mechanical bowel preparation is not necessary. Given the low likelihood of major bleeding, a type and screen are adequate for laparoscopic and robotic procedures.
33.4 Informed Consent
Preoperative counseling involves discussion of risks, benefits and alternatives, including intraoperative risks of urinary tract infection, bleeding requiring transfusion, injury to adjacent structures including the bladder, ureters, rectum, bowel and major vessels, as well as cardiovascular, pulmonary and thromboembolic complications; perioperative complications include incisional hernia, hematoma formation, breakdown of the urethral or bladder neck repair, persistent urine leak with potential fistula formation, recurrent stenosis, rectovesical or rectourethral fistula formation should a rectal injury occur. Patients should be counseled that surgical treatment of the stenosis may lead to post-operative urinary incontinence, necessitating artificial urinary sphincter placement to restore continence.
33.5 Setting Up the Operating Room
33.5.1 Operative Equipment
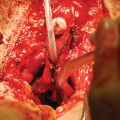
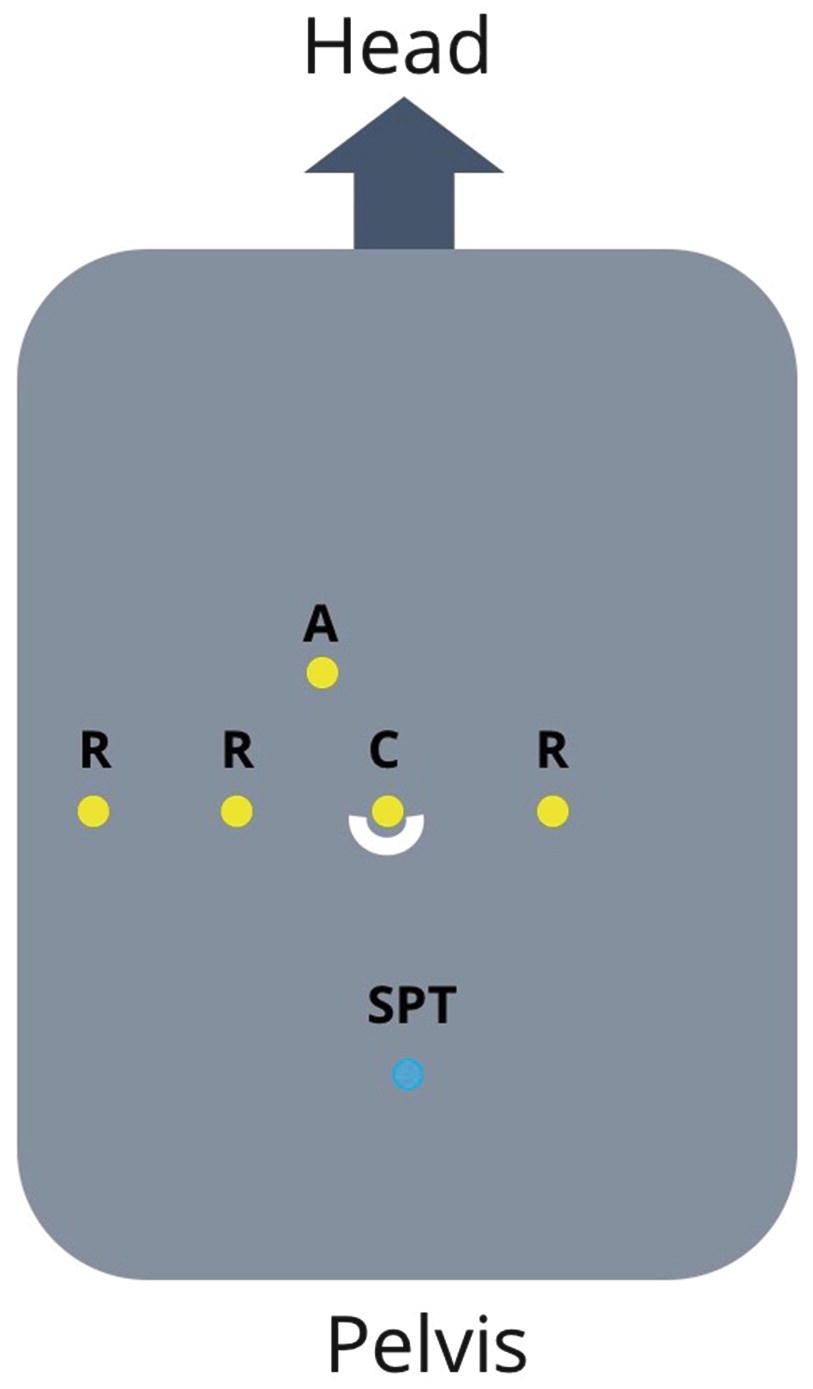
The da Vinci Robotic System (Intuitive Surgical, Sunnyvale, CA) Xi or SP (Single Port) systems are preferred for robotic posterior urethral reconstruction. While we have used the Si system for reconstruction, the Xi system allows for easy side dock for current perineal access. Compared to the Xi, the newer da Vinci SP system offers improved ability to work within a narrow space deep within the pelvis. An AirSeal filtered tube set and access port (ConMed, Utica, NY) allows for maintenance of pneumoperitoneum in cases of concurrent perineal dissection. A flexible cystoscope is used to localize the distal extent of the stricture , and to evaluate the location of the urethral rhabdosphincter.
33.5.2 Patient Positioning and Preparation
After induction of general anesthesia , an orogastric tube should be placed to decompress the stomach. Patients are positioned in low lithotomy for access to the perineum and anus. Arms may be tucked to the sides with padding to avoid median and ulnar nerve injuries. Likewise, the knees, calves and any bony prominences should be padded carefully to prevent excess pressure and neuromuscular complications. The patient should be adequately secured to the table for steep Trendelenberg positioning.
Pneumatic compression stockings are routinely placed, in addition to administration of 5000 units of subcutaneous heparin for patients at moderate to high risk of venous thromboembolism.
Intravenous antibiotics are administered up to one hour prior to the start of the procedure, either with a cephalosporin for negative preoperative urinalysis or an antibiotic targeted to the patient’s urine culture results (Wolf AUA guidelines stewardship).
It is important to maintain close communication between the anesthesiology and surgical teams intraoperatively to prevent or identify complications of carbon dioxide insufflation and pneumoperitoneum.
33.6 Surgical Technique
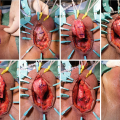
33.6.1 Vesicourethral Anastomotic Stenosis Following Radical Prostatectomy
Pneumoperitoneum is established using either a Veress needle or using an open Hasson technique. CO2 insufflation pressure is typically maintained at 10–5 mm Hg throughout the operation. After initial placement of an 8 mm metal robotic trocar for the camera, our practice is to perform regional transverse abdominis plane (TAP) blocks under visualization. The blocks are administered through the triangle of Petit on each side, depositing local anesthetic in the plane between the internal oblique and transverse abdominis muscles to target the innervation of the anterolateral abdominal wall.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree