7 Reconstruction of the ear
Synopsis
 The commonest acquired ear deformity presenting for reconstruction is the partial ear defect.
The commonest acquired ear deformity presenting for reconstruction is the partial ear defect.
 Apart from acquired ear deformities, microtia is one of the commonest and most complex conditions that present for reconstruction.
Apart from acquired ear deformities, microtia is one of the commonest and most complex conditions that present for reconstruction.
 Patients with microtia may have associated anomalies
Patients with microtia may have associated anomalies
 Reconstruction is usually a staged procedure.
Reconstruction is usually a staged procedure.
 Creation of an adequate cartilage framework is key to the success of reconstruction.
Creation of an adequate cartilage framework is key to the success of reconstruction.
Historical perspective
Ear reconstruction was first referred to in the Susruta Samhita,1 where a cheek flap was suggested for repairing the earlobe. As early as 1597, Tagliacozzi described repair of both upper and lower ear deformities with retroauricular flaps.2 In 1845, Dieffenbach described repair of the ear’s middle third with an advancement flap3 (see Fig. 7.44, below). This may occasionally have application today.
This early work focused mainly on traumatic deformities. However, by the end of the 19th century, surgeons began to address congenital defects, particularly prominent ears.4
The origin of microtia repair had its significant beginnings in 1920, when Gillies buried carved costal cartilage under mastoid skin,5 then separated it from the head with a cervical flap. Ten years later, Pierce modified this method by lining the new sulcus with a skin graft and building the helix with a tubed flap.6 In 1937, Gillies repaired more than 30 microtic ears using maternal ear cartilage7; these were found to resorb progressively.8
Experiencing the same frustration as others,9–12 Steffensen13 used preserved rib cartilage to produce excellent results, but later reported progressive resorption of the same cartilage frameworks.14
A major breakthrough came in 1959, when Tanzer used autogenous rib cartilage, which he carved in a solid block.15 His excellent results have persisted over the years.16
In an effort to circumvent large operations, Cronin (1966) introduced silicone ear frameworks,17 but found that, similar to other inorganic implants, e.g., polyethylene, nylon mesh, Marlex, polyester net, and Teflon, they suffered a high incidence of extrusion.18,19 Initially, Cronin minimized this problem by providing fascia lata or galeal and fascial flaps for extra autogenous rim coverage,20 but later, when he found that the alloplastic frames still extruded, he discontinued this practice.
In spite of some investigators’ enthusiasm for Medpor frameworks,21,22 these foreign substances tend to encounter the same problems that have plagued silicone. Most recently, a patient came to my consultation with an infected sinus tract that had been draining from her Medpor implant for 2 years; another patient presented with three separate exposed areas of Medpor in his reconstructed ear. On the other hand, once safely past the 10th postoperative day, I have never lost an autogenous ear framework in a microtia patient; only one has been lost in a trauma patient with poor compliance with postoperative instructions.23 To date, more than 70 of my reconstructed ears have survived major trauma.
For many years there has been considerable interest in creating a “prefabricated” framework from autogenous cartilage in order to circumvent the necessity to fabricate an ear framework during a prolonged reconstructive procedure, and to attempt to eliminate the variability of the surgeon’s artistic ability to create a realistic ear framework from rib cartilage. Young24 and Peer25 first conceived the idea of “framework prefabrication” prior to the actual auricular reconstruction back in the 1940s. This innovative technique was accomplished by means of “diced” pieces of autogenous rib cartilage which were placed in a fenestrated two-piece, ear-shaped vitallium mold which in turn was banked in the patient’s abdominal wall. After several months they retrieved the banked mold, opened it, and harvested the framework of cartilage chips, which had united by connective tissue which had grown through the mold’s openings. However, the results were not consistent, perhaps because contraction of the fibrous tissue surrounding the multiple cartilage islands distorted the resultant framework.
Interest in this “prefabrication” concept has been rekindled via modern “tissue-engineering” techniques in which cartilage cells are grown in the laboratory and seeded upon a synthetic, biodegradable ear form which is then implanted beneath the skin of a mouse.26 The early results are interesting, but it should be noted that the trial work is not being carried out under the same conditions as in a clinical human ear reconstruction – the investigators’ frameworks are being placed under the very loose skin of an animal’s back, whereas in human ear repair, the new framework must be placed underneath snug skin just in front of the hairline in the ear region.
However, I have long been intrigued with the concept of creating a living “prefabricated” ear framework, and began collaborating with researchers several years ago27 to explore the possibilities of bioengineering firm, autogenous cartilage frameworks, to see if some of the above-mentioned limitations can be overcome.28 Our direction involves growing autogenous costal cartilage in varying-sized molds made from “idealized” frameworks that I have sculpted and cast. But to fill these molds with chondrocytes obtained by digesting a large volume of rib cartilage would merely be reproducing Young and Peer’s work by newer technology. Our goal is to exploit the technology using a small piece of cartilage (perhaps obtained by biopsy from the microtia patient at age 3–4 years, when neochondrogenic potential is high), then efficiently to extract the chondrocytes, expand them in culture, and infuse them into the ideal matricial substrate within the ideal ear framework mold for that specific patient. Once generated to satisfaction, the engineered framework would then be “banked” under the patient’s hairless periauricular skin as the first reconstructive surgical phase. For this technique to be successful, the major problems that must be overcome are replicating sufficient chondrocytes from a small cartilage sample (25–50 million cells/mL are needed for neocartilage formation in a construct, and the ear mold volume is about 5 cc), and the ability of those chondrocytes to regenerate firm cartilage matrix so that the engineered framework can withstand the pressure caused by the constraints of a two-dimensional skin cover that is taut, inelastic, and restrictive.
In my experience, until tissue engineering evolves beyond the aforementioned problems, sculpted autogenous rib cartilage remains the most reliable material which produces results with the least complications.16,23,29–32 Furthermore, rib cartilage provides the most substantial source for fabricating a total ear framework. Although contralateral conchal cartilage has been used for this purpose,33,34 it seems best to reserve auricular cartilage for repairing partial ear defects, for which considerably less tissue bulk is needed.
Anatomy
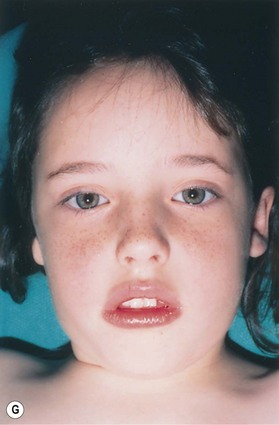
In most microtic vestiges, the presence of this lobule tissue is a valuable asset in the repair (Fig. 7.1; see Fig. 7.13). When the lobule is lost in total ear avulsions, it is best recreated by shaping the bottom of the carved ear framework to resemble the lobe.
Practical embryology and understanding the middle-ear problem
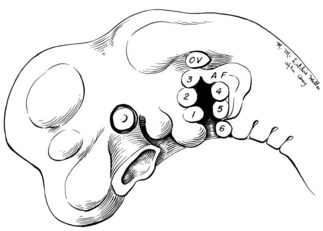
Tissues of both the middle ear and external ear are derived chiefly from the first (mandibular) and second (hyoid) branchial arches. The auricle itself is formed from six “hillocks” of tissue that lie along these arches and can be first seen in the 5-week embryo35–37 (Fig. 7.2).
The inner ear first appears at 3 weeks and is derived from tissues of distinctly separate ectodermal origin. Perhaps this explains why it is usually spared the developmental mishap that almost invariably involves the middle ear of microtic patients. Refinements in radiographic technique (polytomography and computed tomography scans) have only occasionally demonstrated dysplasia and hypoplasia of the inner ear.38,39 Inner-ear abnormalities are found in approximately 10% of microtia and atresia cases, but the abnormalities are usually slight (e.g., a dilatation of the lateral semicircular canal). Interestingly, in evaluating approximately 1500 microtic cases over a 25-year period, the author has seen only three patients who were totally deaf. Remarkably, these were unilateral microtias who had no family history of microtia. Because of their normal inner ear, even patients with bilateral microtia usually have serviceable hearing and use bone-conductive hearing aids to overcome the transmission block. If they are referred to an audiologist so that aids can be used as soon as possible, these patients usually develop normal speech. There is no point in waiting several months. Hearing aids should be applied within weeks of birth. Because these bone-conductive aids are cumbersome and label the child as “different” from his or her peers, it is optimal to correct the hearing deficit surgically to eliminate the aids. However, surgically correcting this conductive problem is difficult as the middle ear beneath the closed skin is not normal.
Exploration involves cautiously avoiding the facial nerve while drilling a canal through solid bone. The tympanum must usually be created with tissue grafts from the temporal fascia; the distorted or fused ossicles may be irreparable. As skin grafts do not take well on the drilled bony canal, chronic drainage is a frequent complication and meatal stenosis is common. Finally, unless the surgeon can close the functional difference between the repaired and normal ear to within 15–20 dB (an elusive feat in most surgeons’ hands), binaural hearing will not be achieved. However, in the hands of a competent otologist with a large volume of experience, restoring middle-ear function surgically can be rewarding – even if only one ear is involved.40,41
When middle-ear surgery is contemplated, a team approach must be planned with an experienced otologist. In these cases, the auricular construction should precede the middle-ear surgery, as once an attempt is made to “open the ear” the virgin skin is scarred, which compromises a satisfactory auricular construction. On the horizon lie implantable acoustic devices, which may offer a solution for these patients. The bone-anchored hearing aid (BAHA) offers even better hearing than a bone-conductive hearing aid for patients who are not candidates for atresia surgery,42,43 but does require two stages of surgery for optimal implantation of the metal posts in the skull.
Etiology
Incidence
According to an extensive study conducted by Grabb,44 microtia occurs once in every 6000 births. The occurrence is estimated at 1 in 4000 in Japanese, and as high as one in 900–1200 births in Navajo Indians.45
Hereditary factors
In a study conducted by Rogers,46 morphologic, anatomic, and genetic interrelationships were shown to exist between microtia, constricted, and protruding ears. In this thorough investigation, Rogers demonstrated that these deformities are interrelated and can be hereditary.
Preauricular pits and sinuses, and a combination of pits, preauricular appendages, cupping deformity, and deafness, are all hereditarily dominant.1,47 Both dominant and recessive characteristics have been revealed in deafness associated with several auricular abnormalities.48 Ear deformities frequently recur in families of mandibulofacial dysostosis (Treacher–Collins syndrome).49 These are frequently constricted ear deformities, an abnormality that is known to be hereditary.12,50–52 Hanhart found a severe form of microtia associated with a cleft or high palate in 10% of family members studied,51 and Tanzer53 found that approximately 25% of his 43 microtia patients had relatives with evidence of the first and second bronchial arch syndrome (craniofacial microsomia); microtia was present in four instances.
In 4.9% of the author’s first 1000 microtia patients, family histories revealed that major auricular deformities occurred within the immediate family, i.e., parents, siblings, aunts, uncles, or grandparents.23 When “distant” relatives were included, the percentage jumped up to 10.3%. In 6% of patients, preauricular skin tags or minor auricular defects were observed in the immediate family. This number also rose to 10.3% when all relatives were included. Immediate family members with normal auricles but underdeveloped jaws or facial nerves were seen in 1.2% of patients.23
In a thorough, intensive survey of 96 families of their 171 microtic patients, Takahashi and Maeda54 ruled out chromosomal aberrations and concluded that inheritance must be multifactorial and that the recurrence risk is 5.7%. In previous studies, others have found multifactorial inheritance between 3 and 8% in first-degree relatives. If a couple has two children with microtia, the risk of recurrence in future offspring is thought to be as high as 15%.
Specific factors
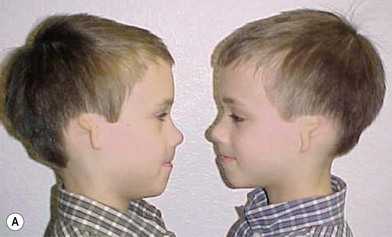
McKenzie and Craig55 and Poswillo56 theorized that the cause of developmental auricular abnormalities is in utero tissue ischemia resulting from either an obliterated stapedial artery or a hemorrhage into the local tissues. This suggests that these deformities arise from a mishap during fetal development rather than from a hereditary source. Interestingly, the author has treated at least 15 microtia patients who have an affirmed identical twin with normal ears.23 Only two sets of the author’s identical twin patients have had concordance for outer-ear deformities; one set were identical mirror-image twins, and the other set both had right-sided microtia. Of interest in the latter set is that each of these male twins with right microtia also had signs and symptoms of pyloric stenosis within 2 days of each other and were operated on at 6 weeks of age (Fig. 7.3).
The occurrence of deafness and occasional microtia resulting from rubella during the first trimester of pregnancy is well known. Also, certain drugs during this critical period may be causative; the author has seen at least 3 cases of microtia which resulted from the mother’s ingestion of the tranquilizer, thalidomide.57,58 Isotretinoin has also been cited as causing ear deformities when ingested during the first trimester.59 In many instances mothers had been unaware of this problem and had used this drug to control acne. Other medications that reportedly cause microtia are clomiphene citrate60 and retinoic acid.61
Diagnosis
Classification
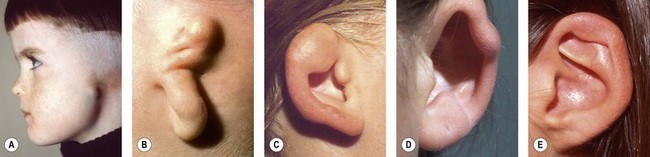
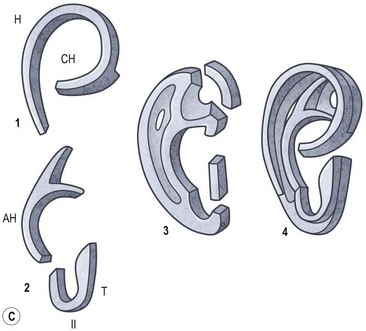
Rogers noted that most types of auricular hypoplasia could be classified in a descending scale of severity.46 This corresponds to Streeter’s depiction of embryological patterns of auricular development.37 Rogers divides developmental ear defects into four groups: (1) microtia; (2) lop ear, i.e., folding or deficiency of the superior helix and scapha; (3) “cup” or constricted ear, with a deep concha and deficiency of the superior helix and antihelical crura; and (4) the common prominent or protruding ear.
Using a system that correlates with embryological development, Tanzer classifies congenital ear defects according to the approach necessary for their surgical correction62 (Box 7.1 and Fig. 7.4).
Box 7.1
Clinical classification of auricular defects (Tanzer)
Associated deformities
Because the auricle develops from tissues of the mandibular and hyoid branchial arches, it is not surprising that a significant percentage of microtic patients exhibit deficient facial components that originate from these embryological building blocks. These deformities are compiled under the heading of “craniofacial microsomia” (first and second branchial arch syndrome), and an obvious facial asymmetry was noted in 35% of the author’s first 1000 microtia patients.23 The most complete genetic expression of this condition includes defects of the external and middle ear; hypoplasia of the mandible, maxilla, zyogomatic, and temporal bones; macrostomia and lateral facial clefts; and atrophy of facial muscles and parotid gland.44,63,64 Furthermore, Brent found that 15% of his 1000 patients had paresis of the facial nerve.23 Dellon et al. have shown that the palatal muscles are rarely spared in this syndrome.65
Urogenital tract abnormalities are increased in the presence of microtia,66 particularly when the patient is afflicted with other manifestations of the first and second branchial arch syndrome.67 The associated deformities found in the author’s first 1000 microtia patients are compiled in Table 7.1.
Microtia
Clinical characteristics
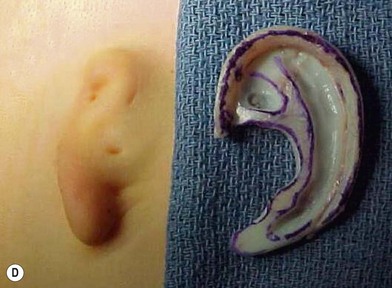
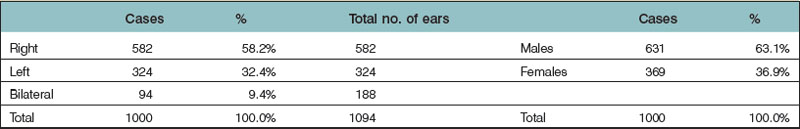
Microtia varies from the complete absence of auricular tissues (anotia) to a somewhat normal but small ear with an atretic canal. Between these extremes there are an endless variety of vestiges, the most common being a vertically oriented sausage-shaped nubbin (Figs 7.1, 7.5, and 7.6). Microtia is nearly twice as frequent in males as in females, and the right–left–bilateral ratio is roughly 6 : 3 : 1 (Box 7.2).23,29,68
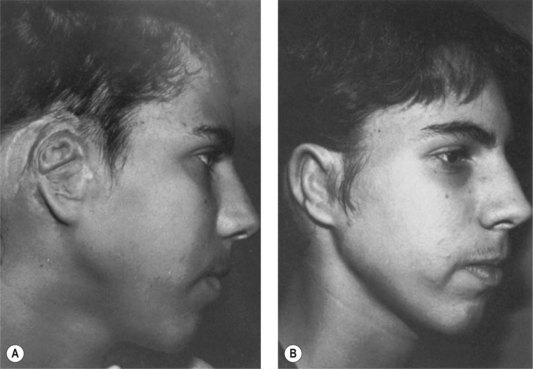
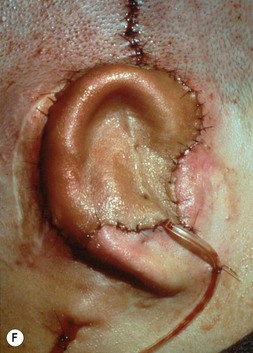
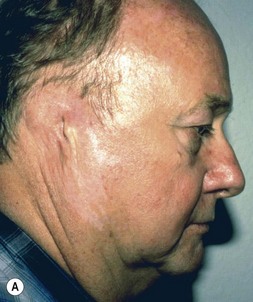
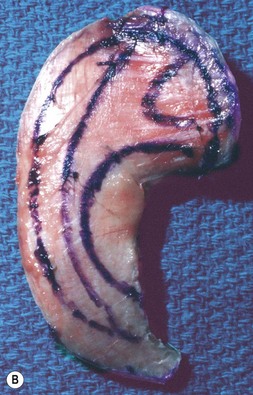
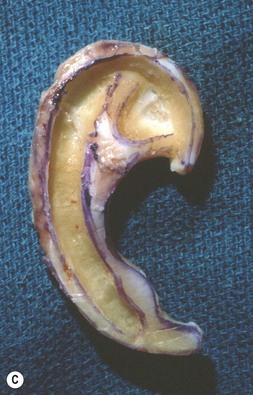
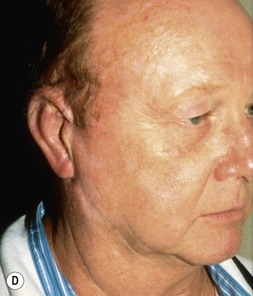
Fig. 7.6 Secondary ear reconstruction with a rib cartilage graft surfaced with a superficial temporoparietal fascial flap and skin graft. (A) A patient with a scarred auricular region after multiple failed procedures. (B) Result achieved in one surgical stage with rib cartilage graft and fascial flap by the techniques outlined in Figure 7.5.
In most instances, the microtic lobule is displaced superiorly to the level of the opposite, normal side, although incomplete ear migration occasionally leaves it in an inferior location. Approximately one-third to one-half the patients exhibit gross characteristics of craniofacial microsomia, although Converse and associates have demonstrated tomographically that skeletal deficiencies exist in all cases.69,70 Whatever the deformity, the author has been impressed with its potential for psychological havoc amongst the entire family, varying from the patient’s emotional insecurity to the parents’ deep-seated guilt feelings.
General considerations
The age at which an auricular construction should begin is governed by both psychological and physical considerations. Since the body image concept usually begins forming around the age of 4 or 5 years,71 it would be ideal to begin construction before the child enters school, and before he/she is psychologically traumatized by his/her peers’ cruel ridicule. However, surgery should be postponed until rib growth provides substantial cartilage to permit a quality framework fabrication.
At age 6, the normal ear has grown to within 6 or 7 mm of its full vertical height,72 which permits construction of an ear that will have reasonably constant symmetry with the opposite normal ear. In his follow-up mail survey, Tanzer16,73 found that an ear constructed from autogenous rib cartilage grows at the same rate as the normal ear, with the possible exception of patients operated at 6–7 years of age, where he noted that 50% of these ears lagged several millimeters behind in growth; the roles played in the growth of his patients’ surgically constructed ears by soft tissues and by cartilage have not been determined. In evaluating my own patients29 operated on between ages 5 and 10 with a minimum 5-year follow-up (76 patients), I found that 48.1% of the constructed ears grew at an even pace with the opposite, normal ear; 41.6% grew several millimeters larger; and 10.3% lagged several millimeters behind the normal side. From the limited number of long-term patients in this age group whom I have examined, I found that most constructed ears have grown, and many of these have not only kept up with the little residual growth in the opposite ear, but have slightly overgrown29. I found none to have shrunk, softened, or lost their detail .With all this in mind, I conclude that one should try to match the opposite side during the preoperative planning session, regardless of age. Certainly there is no reason to construct the ear larger, as some investigators have previously thought, and one might even consider making the framework several millimeters smaller in the youngest patients where normal, in situ costal cartilage can be expected to exceed normal auricular cartilage growth.
Author’s method of repair
Staging the auricular construction
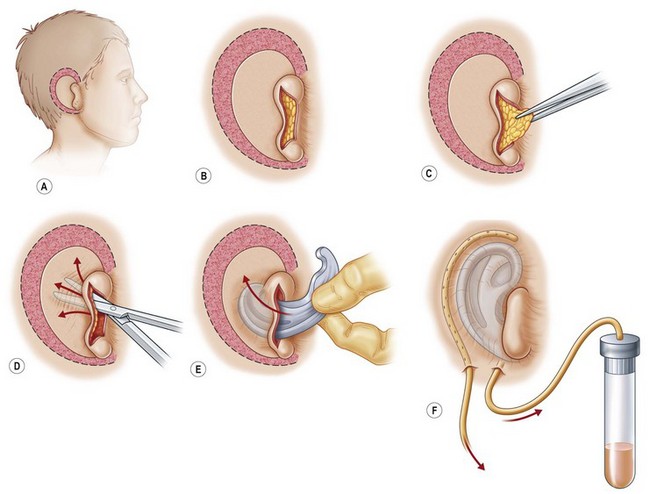
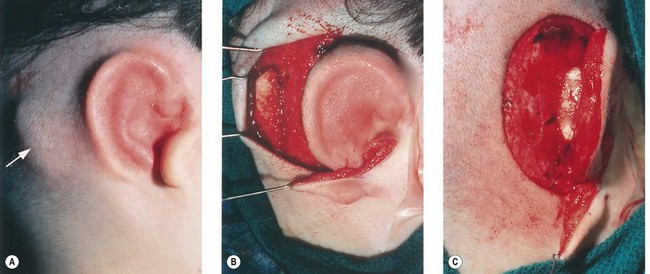
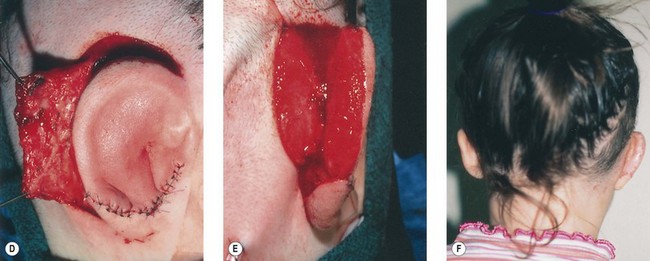
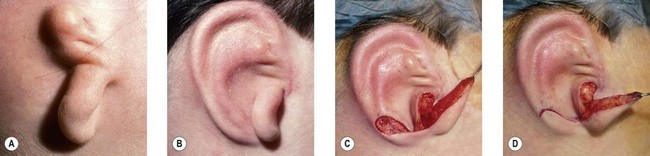
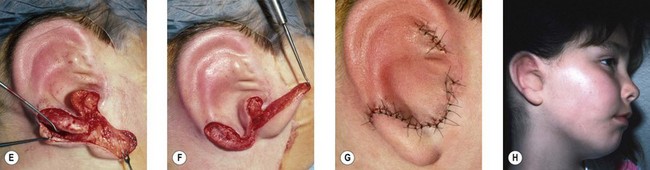
The cartilage graft is the “foundation” of an auricular construction and, as in constructing a house, it should be built and well established under ideal conditions before further stages or refinements are undertaken. By implanting the cartilage graft as the first surgical stage, one takes advantage of the optimal elasticity and circulation of an unviolated, virgin skin “pocket.” For these reasons, I prefer to avoid initial lobule transposition or vestige division, since the resulting scars cannot help but inhibit the circulation and restrict the skin’s elasticity. This, in turn, diminishes its ability to accommodate safely a three-dimensional cartilage graft. It seems easier both to judge the earlobe’s placement and to “splice” the lobule correctly into position with reference to a well-established, underlying framework. Although Tanzer transposed the lobule simultaneously with implantation of the cartilage graft during the last three cases of his clinical practice74 and Nagata routinely does this in his technique,32 the author finds that there is far less risk of tissue necrosis and it is far more accurate to transpose the lobule as a secondary procedure75–77 (Fig. 7.7). The lobe can be safely transposed and simultaneously the ear can be elevated from the head with skin graft if the earlobe vestige is short, because its small wound closure will not compromise the ear’s anterior circulation23,29 (Fig. 7.8).
Planning, preparation, and correlation with the correction of other facial deficiencies
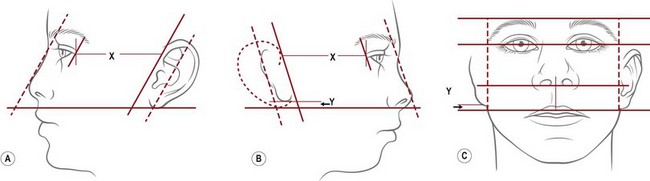
The reconstructed ear’s location is predetermined by first noting the topographical relationship of the opposite, normal ear with facial features and then duplicating its position at the proposed reconstruction site. First, the vestige’s height is compared from the front view with that of the opposite, normal ear. From the side, it should be noted that the ear’s axis is roughly parallel to the nasal profile34,78 (Fig. 7.9). Finally, one notes and records the distance between the lateral canthus and the normal ear’s helical root. Typically, this distance is about 65–67 mm in a 6-year-old child.
When both auricular construction and bony repairs are planned, then careful, integrated timing is essential. Most often the family pushes for the ear repair to begin first, which helpfully assures the auricular surgeon virginal, unscarred skin. The craniomaxillofacial surgeon argues that by going first s/he will correct the facial symmetry, thus making ear placement easier.79 I find this unnecessary when the guidelines described above are followed.
First stage of reconstruction
Obtaining the rib cartilage
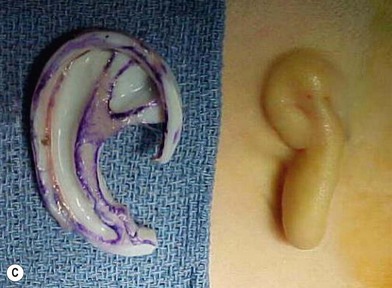
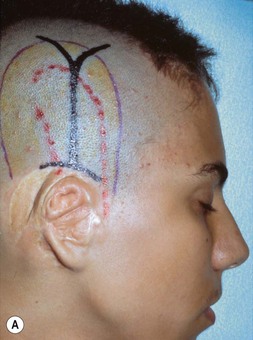
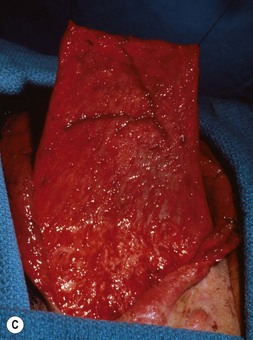
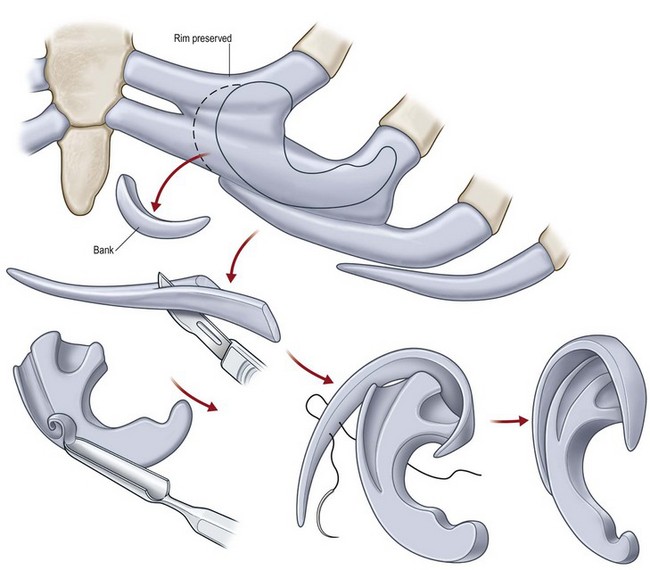
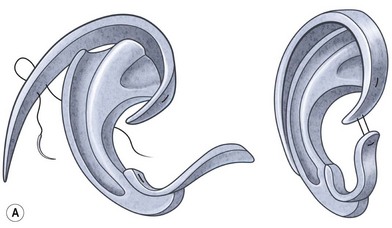
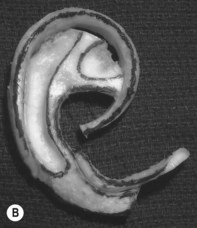
The helical rim is fashioned separately with cartilage from the first free-floating rib (Fig. 7.10). Excision of this cartilage facilitates access to the synchondrotic region of ribs 6 and 7, which supplies a sufficient block to carve the framework body. Extraperichondrial dissection is preferable in obtaining an unmarred specimen. I find that well-documented chest deformities80,81 can be significantly decreased by preserving even a minimal rim of the upper margin of the sixth rib cartilage where the basic shape of the ear is obtained (Fig. 7.10). This precautionary measure retains a tether to the sternum so that the rib does not flare outward to distort the chest as the child grows. If the synchondrotic region seems inadequate in width, one can compensate for framework width by bowing the helix away from the framework body (the “expansile” design82), rather than violating the sixth-rib margin and sacrificing chest wall integrity.
Framework fabrication
The basic ear silhouette is carved from the previously obtained cartilage block (Fig. 7.10). It is necessary to thin very little, if any, of the basic form for a small child’s framework, but it is essential for framework fabrication in most older patients. When thinning is necessary, care should be taken to preserve the perichondrium on the lateral, outer aspect of the framework to facilitate its adherence and subsequent nourishment from surrounding tissues.
Because warping must be taken into consideration,83 the cartilage is sculpted and thinned to cause a deliberate warping in a favorable direction. This allows one to produce the acute flexion necessary to create a helix, which is fastened to the framework body with horizontal mattress sutures; the knots are buried on the frame’s undersurface. For this, I prefer 4-0 clear nylon suture material, which causes only few problems in contrast to the frequent extrusions encountered when using stainless-steel wire sutures for this purpose.
Framework modifications in older patients
Adult rib cartilages are often fused into a solid block, which invites one to sculpt the ear framework in one piece – not unlike doing a wood carving (Fig. 7.11). In my experience, this is advantageous because adult cartilage is often calcified; it is difficult, if not impossible, to create a separate helix that will bend without breaking. If a one-piece carving produces insufficient helical projection, the helix can be detached and slid up the framework body to augment the protrusion of the rim. This improved contour is maintained by reattaching the helix to the framework with several permanent sutures (Fig. 7.12).
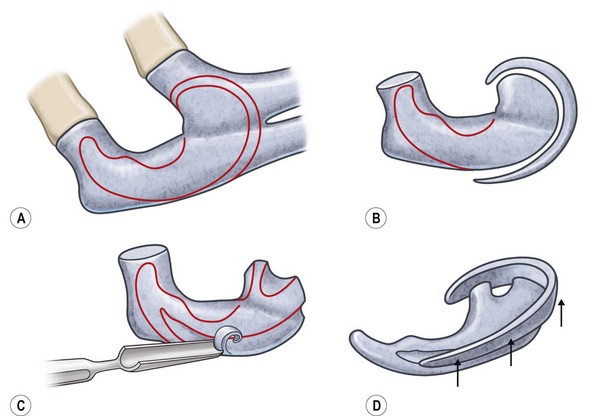
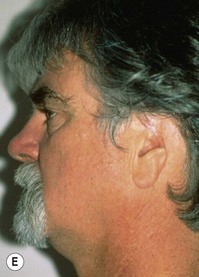
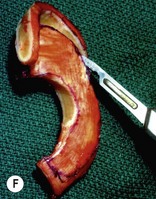
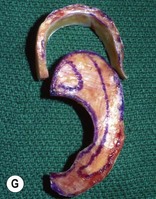
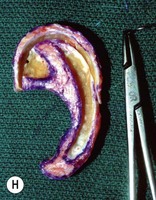
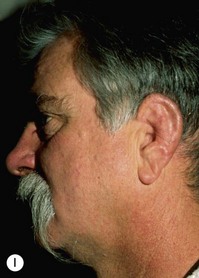
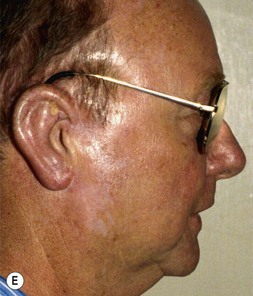
Fig. 7.12 Maximizing rim projection in adult patient with sliding helical advancement. (A,B) Harvesting fused rib cartilage block and separating the inflexible helical portion; (C) sculpting body of framework; (D) sliding and reattaching the helix to maximize its projection. (E) A 50-year-old man with ear loss from a dog bite. (F–H) Construction of the framework by use of the technique illustrated in the drawings. (I) The completed repair. This patient had his hairline “idealized” by laser treatment before the rib cartilage graft (see Fig. 7.20).
(Reproduced from Brent B. Technical advances in ear reconstruction with autogenous rib cartilage grafts: personal experience with 1200 cases. Plast Reconstr Surg. 1999;104:319.)
Framework implantation
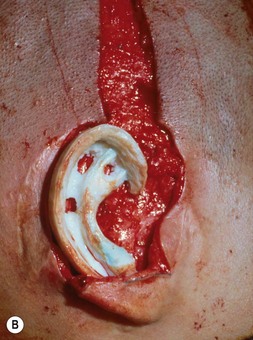
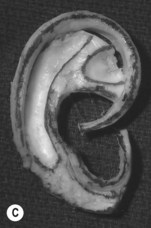
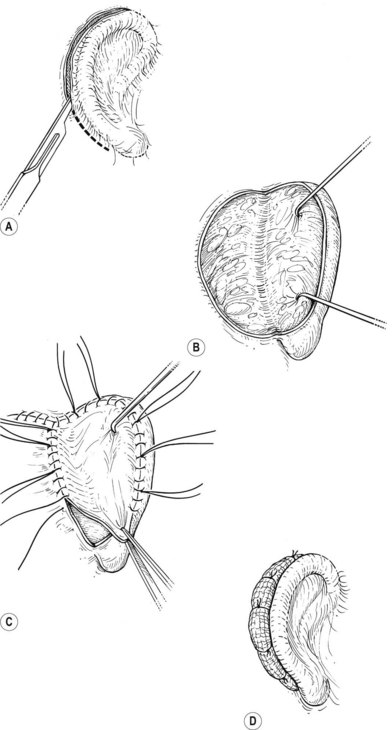
A cutaneous pocket is created with meticulous technique so as to provide an adequate recipient vascular covering for the framework. Through a small incision along the backside of the auricular vestige, a thin flap is raised by sharp dissection, taking care to preserve the subdermal vascular plexus. So as to evaluate the flap’s vascular status and to assure accurate hemostasis, epinephrine-containing solutions are avoided. With great care, the skin is dissected from the gnarled, native cartilage remnant which then is excised and discarded. Finally, the pocket is completed by dissecting a centimeter or two peripherally to the projected framework markings (Fig. 7.1).
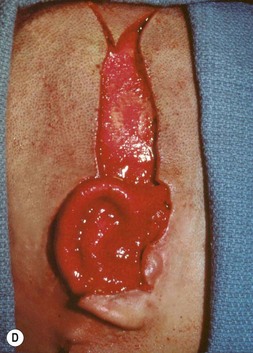
Insertion of the framework into the cutaneous pocket takes up the valuable skin slack which was created when the native cartilage remnant was removed. The framework displaces this skin centrifugally in an advantageous posterosuperior direction so as to displace the hairline just behind the rim. This principle of anterior incision and centrifugal skin relaxation, introduced by Tanzer,15 not only permits advantageous use of the hairless skin cover, but also preserves circulation by avoiding incisions and scars along the helical border.
Although Tanzer initially suggested the use of bolster sutures to coapt the skin flap to underlying framework,15,84 the author finds it far safer to do this with suction, which simultaneously prevents fluid collection and minimizes the risk of flap necrosis along the helical margin.
To attain skin coaptation via suction, silicone catheters are used with the needles inserted into rubber-topped vacuum tubes, the tubes being retained on a rack to observe changes in quantity and quality of drainage. Although a dressing is applied which accurately conforms to the convolutions of the newly created auricle, firm pressure is dangerous and unnecessary, and must be avoided. Hemostasis and skin coaptation are provided by the suction drain.17,29,75,77
Immediate postoperative care and management of complications
Immediately upon suspecting an infection, an irrigation drain is introduced below the flap, and continuous antibiotic drip irrigation is begun. Appropriate adjustments are made in both the antibiotic drip irrigation and in the systemic therapy when sensitivities are available from the initial culture. Cronin17 has salvaged Silastic-frame reconstructions impressively by this technique, and I had success in managing the rare infection in cartilage graft reconstructions when I was using bolster sutures during my first 15–20 cases. The problem of infections basically disappeared when I switched over to the suction drain system and I have not experienced one in an autogenous cartilage framework for nearly 30 years.
Postoperative activities and care
The ear itself withstands trauma well, since, like the opposite, normal ear, it houses a framework of autogenous cartilage. To date, the author has witnessed numerous reconstructed ears undergo traumatic episodes, e.g., baseball and soccer blows, a bee sting, and a dog bite. They have all healed well.29 For these reasons, the author recommends no conspicuous, protective headgear, except in sports such as football and wrestling, where such equipment is used routinely with normal ears.
Other stages of auricular construction
Rotation of the lobule
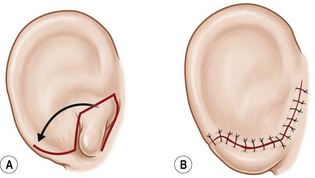
The “rotation” or repositioning of this normal but displaced structure is accomplished essentially by Z-plasty transposition of a narrow, inferiorly based triangular flap (Fig. 7.13).
Nagata32 and Firmin85 transpose the earlobe and utilize skin from the lobule’s posterior surface to line the framework’s tragal strut during the first-stage surgery. This does produce an excellent tragal appearance, but the price paid is greater risk of skin necrosis, and earlobes which are at times compromised in appearance and often unable to accommodate earrings. This latter problem is no small issue for my young female patients, who often submit to surgery with eventual earlobe piercing as their highest priority.23
If the lobe vestige is short so that a substantial skin bridge can be preserved above it during its transposition, then the earlobe can safely be moved while simultaneously separating the auricle from the head to create the auriculocephalic sulcus23,29 and preserve sufficient posterior earlobe skin to permit the use of earrings (Fig. 7.8).
Tragal construction and conchal definition
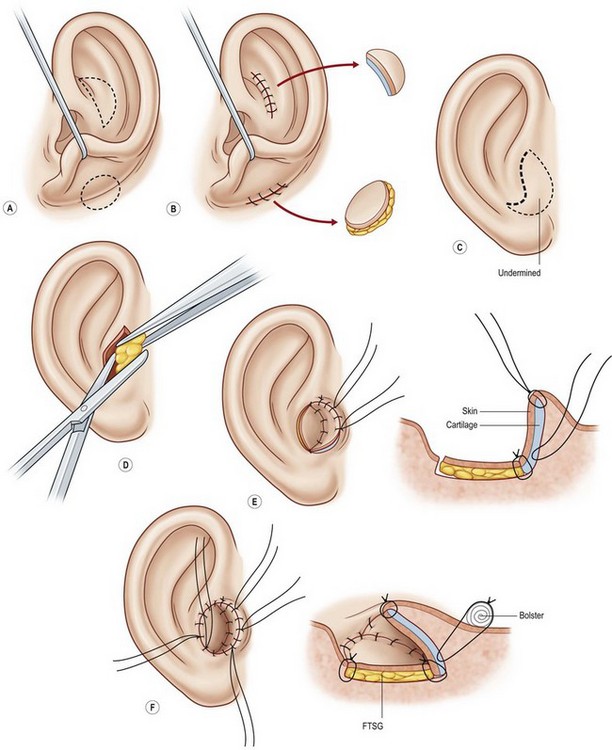
One can form the tragus, excavate the concha, and mimic a canal in a single operation. This is accomplished by placing a thin elliptical-shaped chondrocutaneous composite graft beneath a J-shaped incision in the conchal region75,77 (Fig. 7.14). The main limb of the “J” is placed at the proposed posterior tragal margin; the crook of the “J” represents the intertragal notch. Extraneous soft tissues are excised beneath this tragal flap to deepen the concha; this excavated region looks quite like a meatus when the newly constructed tragus casts a shadow upon it.
I also use an alternative method of creating the tragus as an integral strut of the original framework during its initial fabrication. I achieve this with a small piece of rib cartilage which is first fastened to the frame so as to create the antitragal eminence. The strut is thinned on one side, then curved around and affixed by its distal tip with a clear nylon suture which stretches across to the frame’s crus helix. The result is a delicate tragus which flows naturally from the main framework through an arched intertragal notch (Fig. 7.15). This method of tragus construction is particularly advantageous in bilateral microtia where there is no source for special composite tissue grafts. Nagata also creates the tragus with an extra cartilage piece attached to his main framework (Fig. 7.16), and lines it with skin from the earlobe vestige during the first stage of surgery.
(Redrawn from Spina V, Psillakis JM. Total reconstruction of the ear in congenital microtia. Plast Reconstr Surg. 1971;48:349.)
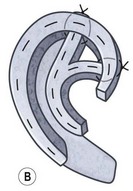
Another option for tragus construction in bilateral microtia is the modified Kirkham method, which consists of an anteriorly based conchal flap doubled on itself.11
Detaching the posterior auricular region
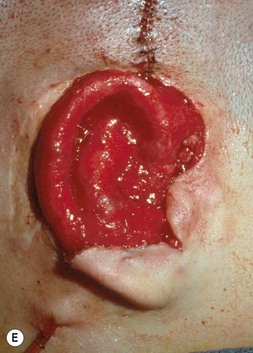
The posterior auricular margin is defined by separating the ear from the head and covering its undersurface with a skin graft. In the past, I used a thick split graft but now strictly use full-thickness skin grafts. This stage of surgery should not be attempted until the edema has markedly subsided and the auricular details have become well defined. This surgery is initiated by making an incision several millimeters behind the rim, taking care to preserve a protective connective tissue layer on the cartilage framework. The retroauricular skin is then advanced into the newly created sulcus so that the only graft requirement is on the ear’s undersurface. I harvest a full-thickness skin graft from the lower abdomen or groin crease and suture it to the wound with the sutures left long; these are tied over a bolster to tamponade the graft to the recipient bed (Fig. 7.17).
Greater projection of the auricle can be achieved by placing a wedge of rib cartilage behind the elevated ear, but this must be covered with a tissue flap in order for the skin graft to take over the cartilage. Nagata32 accomplishes this with an axial flap of temporoparietal fascia (Fig. 7.18). However, use of that fascial flap is not without a certain morbidity, and I feel that this fascia should be reserved for significant traumatic and secondary reconstruction cases where there is no other option for their solution.29 Like Firmin85 and Weerda,86 I prefer to cover the cartilage wedge with a turnover “book flap” of occipitalis fascia from behind the ear23 (Fig. 7.8). When this cartilage-wedging technique is used, there is no need to subject the patient to a second uncomfortable chest operation by harvesting rib cartilage anew, as does Nagata.87 Instead, an extra piece of cartilage can be banked underneath the chest incision during the initial first-stage procedure. When the wedge is needed during the elevation procedure, it can be easily retrieved by incising through the original chest scar. Alternatively, I have also banked this cartilage wedge underneath the scalp, just posterior to the main pocket where the completed ear framework is placed23 (Fig. 7.8A). This site is particularly advantageous in that the nearby banked cartilage can more conveniently be retrieved when later lifting the new ear from the head; furthermore, this scalp site seemingly provides better nourishment for the banked cartilage than does the subcutaneous chest region.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree