10 Reconstruction of the chest
Synopsis
 Rigid chest wall support may be achieved with mesh, acellular dermal matrix, or autogenous material such as tensor fascia lata. Of these, alloplastic mesh is most prone to infection.
Rigid chest wall support may be achieved with mesh, acellular dermal matrix, or autogenous material such as tensor fascia lata. Of these, alloplastic mesh is most prone to infection.
 Soft tissue coverage can be achieved with local muscle flaps.
Soft tissue coverage can be achieved with local muscle flaps.
 Proper treatment of mediastinitis includes debridement, rigid sternal fixation when possible, and soft tissue coverage.
Proper treatment of mediastinitis includes debridement, rigid sternal fixation when possible, and soft tissue coverage.
 Pectoralis muscle is the workhorse for sternal and anterior chest wall defects.
Pectoralis muscle is the workhorse for sternal and anterior chest wall defects.
 Latissimus muscle is known for its bulk and ability to reach intrathoracic defects. Caution is advised for patients with previous thoracotomy incisions as it may have been divided.
Latissimus muscle is known for its bulk and ability to reach intrathoracic defects. Caution is advised for patients with previous thoracotomy incisions as it may have been divided.
 Muscle supplies less bulk than the latissimus but will function to cover lateral chest wall defects and some intrathoracic needs.
Muscle supplies less bulk than the latissimus but will function to cover lateral chest wall defects and some intrathoracic needs.
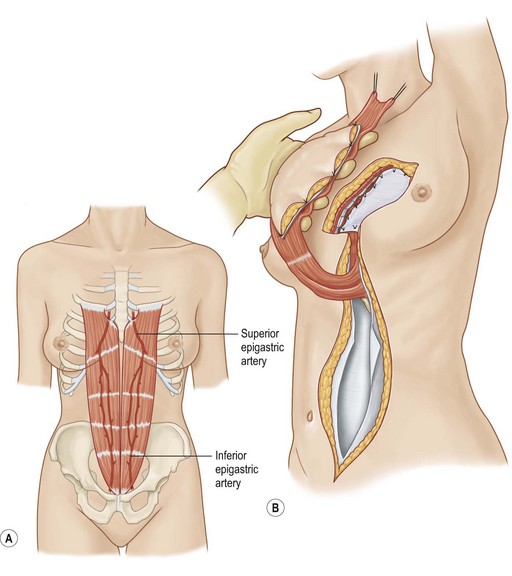
 Rectus abdominus is an excellent choice for sternal and anterior chest wall defects, especially the lower two-thirds. Furthermore, it can be used to fill space within the mediastinum.
Rectus abdominus is an excellent choice for sternal and anterior chest wall defects, especially the lower two-thirds. Furthermore, it can be used to fill space within the mediastinum.
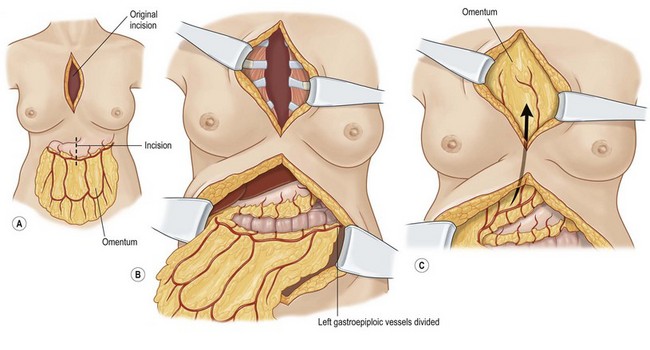
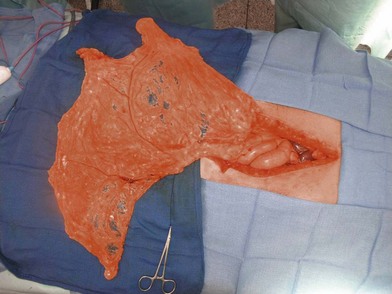
 The omentum can reach almost any chest wall defect. Its greatest advantage is its pedicle length, which can be extended by dividing the arcades. It does, however, require a laparotomy for harvest.
The omentum can reach almost any chest wall defect. Its greatest advantage is its pedicle length, which can be extended by dividing the arcades. It does, however, require a laparotomy for harvest.
Introduction
Chest wall reconstruction can be generalized to include skeletal support and soft tissue cover. Skeletal support to prevent paradoxic chest wall motion is usually required when the defect exceeds 5 cm in diameter. Generally, this corresponds to those defects exceeding a two rib resection. This rule of thumb, however, is somewhat region dependent (Table 10.1). Posterior chest wall defects may tolerated up to twice the size of those in the anterior and lateral chest due to scapular coverage and support1,2 Anecdotally, patients who have undergone radiation and have decreased chest wall compliance will tolerate larger resections without skeletal replacement due to an overall fibrosis of their viscera.
Table 10.1 Regions of the chest wall
| Anterior | Between anterior axillary lines |
| Lateral | Between anterior and posterior axillary lines |
| Posterior | Between posterior axillary lines and the spine |
Options for skeletal support include various mesh products including PTFE (Gore-Tex®), polypropylene, Mersilene (polyethylene-terephthalate)/methylmethacrylate,3 and acellular dermal matrix (Fig. 10.1). Furthermore, use of TFL as both graft and flap reconstruction has been described. Little data exists as to outcome comparisons between these options. However, in a retrospective review of 197 patients, PTFE and polypropylene appear to be equivalent in complications and outcomes.1 Another smaller retrospective review of 59 patients prefers Mersilene-methylmethacrylate sandwich to PTFE due of decreased paradoxic chest wall motion.4 As alloplastic implants trend towards an increased infection rate when compared with autogenous material or acellular dermal matrix, the authors prefer to avoid mesh when possible.
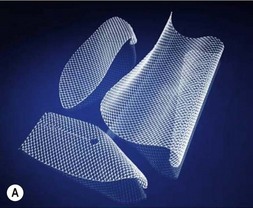
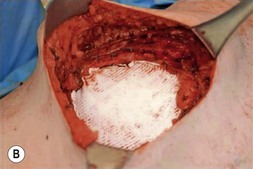
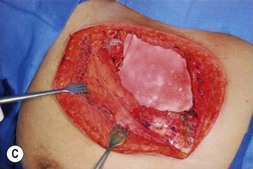
Fig. 10.1 Implantable mesh products including polypropylene, PTFE (Gore-Tex®), and acellular dermal matrix.
Recruitment of local muscles with or without overlying skin is often the first-line of reconstructive offense. These muscles include pectoralis major, latissimus dorsi, serratus anterior, and rectus abdominus. The omentum may also be used. Commonly the ipsilateral latissimus muscle is divided during thoracotomy incisions and the authors encourage early communication between surgeons if there are multiple teams in order to mitigate against routine division. Muscle sparing thoracotomies help to preserve both the latissimus and serratus muscles while providing adequate intrathoracic access (Fig. 10.2).
Common flaps for reconstruction
Pectoralis major
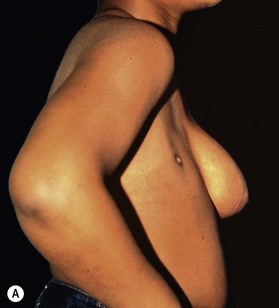
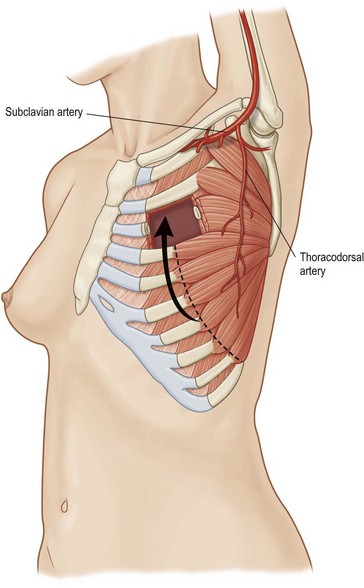
Pectoralis major, a muscle overlying the superior portion of the anterior chest wall, is the workhorse for chest wall reconstruction, especially for defects of the sternum and anterior chest. Its main function is to internally rotate and adduct the arm. Additionally, this muscle serves as the foundation for the female breast and when absent, such as in Poland’s syndrome, reconstruction may be indicated for aesthetic reasons (Fig. 10.3). It originates from the sternum and clavicle and inserts along the superomedial humerus in the bicipital groove. Its dominant pedicle is the thoracoacromial trunk which enters the undersurface of the muscle below the clavicle at the junction of its lateral and middle third. Segmental blood supply is derived from internal mammary artery (IMA) perforators. Based on the thoracoacromial blood supply, it will easily cover sternal and anterior chest wall defects as an island or advancement flap. Division of the pectoralis major muscle insertion can also aid in advancing the muscle flap into a properly debrided mediastinal wound. The muscle can also be turned over based on the IMA perforators and with release of its insertion, cover sternal, mediastinal, and anterior chest wall defects. Importantly, when used as a turnover flap, the internal mammary vessels and their perforators must be examined and deemed intact particularly in the setting of post-sternotomy mediastinitis. This vessel may be absent (left more commonly used than right) due to harvest for coronary artery bypass grafting or damaged during wide debridement of a post-sternotomy wound. The muscle may also be placed intrathoracically, however, this will necessitate resection of a portion of the 2nd, 3rd, or 4th rib (Fig. 10.4). The muscle may be harvested with or without a skin paddle. Donor site deformity including scar placement and loss of anterior axillary fold may be aesthetically displeasing.5
Latissimus dorsi
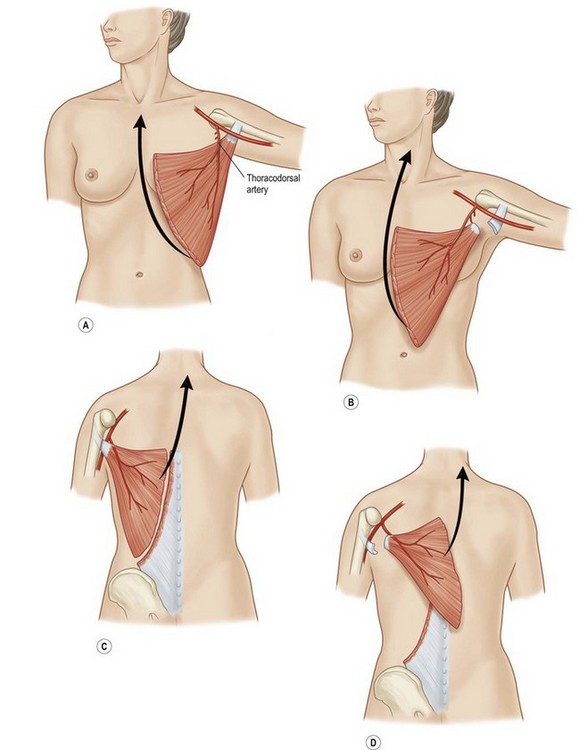
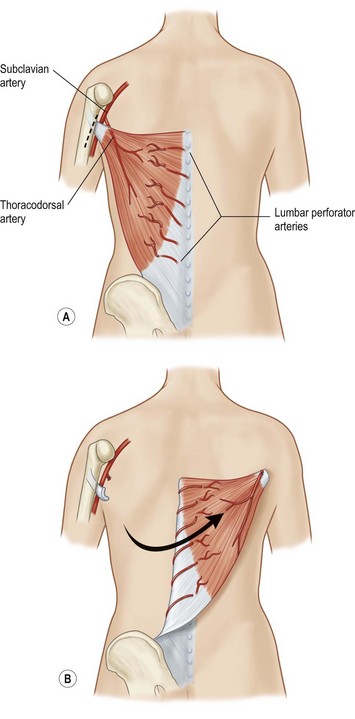
Latissimus dorsi, a large, flat muscle covering the mid and lower back is often recruited for chest wall reconstruction especially when significant bulk and mobility is required. It is easily placed into the chest for intrathoracic space-filling. It is known as the climbing muscle and adducts, extends, and internally rotates the arm. It originates from the thoracolumbar fascia and posterior iliac crest and inserts into the superior humerus at the intertubercular groove. Superiorly, it is attached to the scapula and care must be taken to carefully separate this muscle from the serratus at this point to avoid harvesting both muscles. Its dominant blood supply is the thoracodorsal artery which enters the undersurface of the muscle five centimeters from the posterior axillary fold.6 Segmental blood supply is derived from the posterior intercostals arteries as well as the lumbar artery. Based upon its thoracodorsal pedicle, the muscle can easily reach the ipsilateral posterior and lateral chest wall, including those defects involving either the anterior chest wall, sternum, or mediastinum. It can also be turned over and based upon the lumbar perforators. In this fashion, it can reach across the midline back. Again, it can be moved intrathoracically with rib resection. Donor site morbidity can include shoulder dysfunction, weakness and pain, as well as unattractive scarring.7 However, our experience suggests these concerns are minimal. Also, transposition of this muscle can blunt or obliterate the posterior axillary fold, resulting in some asymmetry (Figs 10.5, 10.6).5 Care must be taken to properly drain the donor site, as seromas are common. Quilting or progressive tension sutures may mitigate against seroma formation.
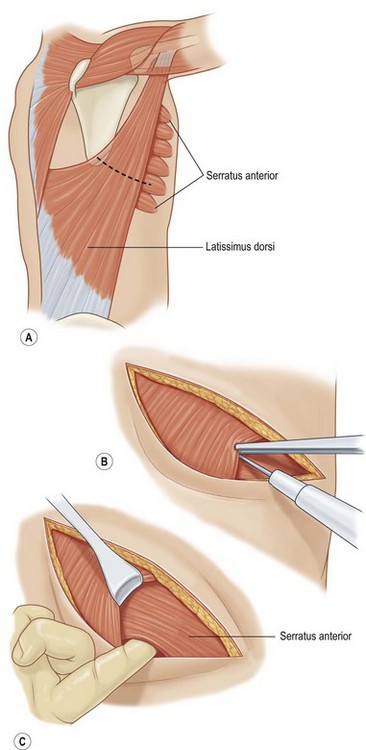
Serratus anterior
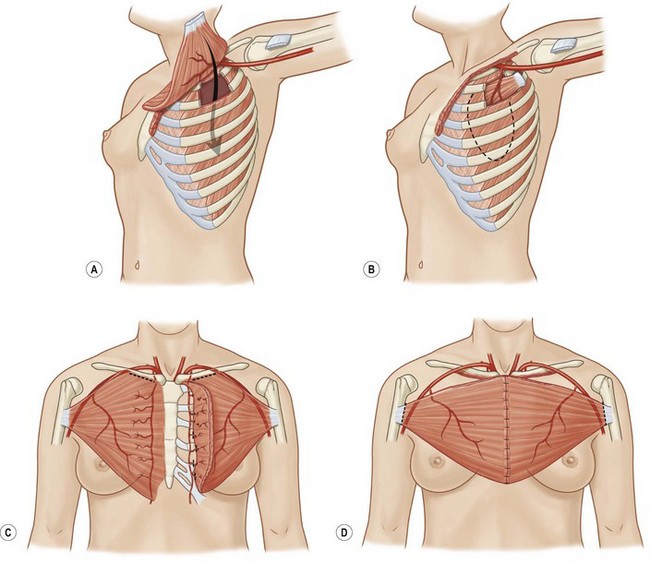
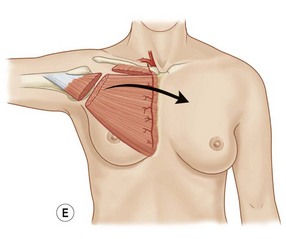
Serratus anterior is a thin broad multi-pennate muscle lying deep along the anterolateral chest wall. It originates from the upper 8 or 9 ribs and inserts on the ventral-medial scapula. It functions to stabilize the scapula and move it forward on the chest wall such as when throwing a punch. It has two dominant pedicles including the lateral thoracic and the thoracodorsal arteries. Division of the lateral thoracic pedicle will increase the arc posteriorly and similarly division of the thoracodorsal will increase the arc anteriorly. The muscle will reach the midline of the anterior or posterior chest. More commonly, however, it is used for intrathoracic coverage, again requiring rib resection. An osteomyocutaneous flap may be harvested by preservation of the muscular connections with the underlying ribs. Donor site morbidity is related to winging of the scapula and can be avoided if the muscle is harvested segmentally and the superior five or six digitations are preserved (Fig. 10.7).5
Rectus abdominus
Rectus abdominus is a long, flat muscle which constitutes the medial abdominal wall. It originates from the pubis and inserts onto the costal margin. It can easily cover sternal and anterior chest wall defects and can also fill space within the mediastinum. It has two dominant pedicles, the superior and inferior epigastric arteries and functions to flex the trunk. With division of the inferior pedicle, the muscle will cover the mediastinum and the anterior chest wall. It may be utilized despite previous IMA harvest based upon its minor pedicle, the 8th intercostals artery. It can be harvested with overlying skin paddle and usually the resulting cutaneous defect can be closed primarily. When taken with overlying fascia, there is a risk for resultant hernia, and at times, mesh reinforcement of the abdominal wall is necessary. Caution is also advised for patients with prior abdominal incisions as the skin perforators or intramuscular blood supply may have been previously violated (Fig. 10.8).5
Omentum
The omentum is comprised of visceral fat and blood vessels which arises from the greater curve of the stomach and is also attached to the transverse colon. This flap can easily cover wounds in the mediastinum, anterior, lateral and posterior chest wall. It has two dominant pedicles, the right and left gastroepiploic arteries. The greatest benefit of this flap is the pedicle length, which can be easily elongated with division of internal arcades. The flap is mobilized onto the chest or into the mediastinum through the diaphragm or over the costal margin. Ideally, the flap is mobilized through a cruciate incision in the right diaphragm as the liver helps to buttress the incision and prevent diaphragmatic hernia. Furthermore, right-sided transposition obviates the need to navigate the flap around the heart. Care must be taken when interpolating the omentum as it is often of very little substance and can easily be avulsed during passage through the diaphragm. Strategies to protect the omentum during transposition include placing the omentum into a bowel bag. The empty bag can be passed from the mediastinum into the abdomen via the diaphragm incision, past the left lobe of the liver. The omentum is then gently packed into the bowel bag with tension transferred to the bowel bag rather than the omentum during interpolation. Caution is again advised for patients with prior laparotomy incisions as the omentum may have significant intra-abdominal adhesions or have been previously resected (Figs 10.9–10.11).5
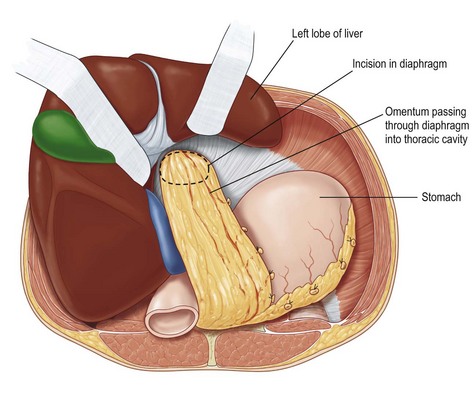
Fig. 10.10 Omentum is passed through cruciate incision in diaphragm under the left lobe of the liver.
History
Despite adversity, however, and as early as 1906, the latissimus dorsi was used for chest wall coverage following radical mastectomy.8 This was similarly performed by Campbell in 1950.9 The earliest use of fascia lata grafts appears in 1947.10 Axially-based flaps regained popularity in the 1970s and in 1986, Pairolero and Arnold published their series of 205 patients managed with muscle flaps purporting their safety and durability.11
As surgical advances and innovations were made, the sequelae of postoperative infection followed close behind. Interestingly, the treatment of mediastinal infection has changed dramatically since the first description of the sternotomy incision in 1957.12 Open chest drainage fell out of favor quickly due to exposure of heart and mediastinum and subsequent risk of rupture. Mortality rates were as high as 50% with open packing.13 Throughout the 1960s, closed chest drainage with antibiotic catheter irrigation was advocated as the first-line therapy for deep sternal wound infections.14,15 This innovative technique reduced mortality to approximately 20%.16 Then, in 1980, Jurkiewicz published a landmark paper revealing debridement and muscle flap coverage was significantly more successful than antibiotic catheter drainage alone.17 This advancement further reduced mortality rates to 10%.18 In recent times, mediastinitis treatment has advanced to include subatmospheric pressure wound therapy and rigid fixation of residual sternal bone. These techniques address the loss of chest wall integrity, paradoxic chest wall motion, and chronic pain.19,20