Radical Mastectomy
Kirby I. Bland
Introduction
The origin of the Halstedian procedure was one of an oncologically designed procedure that embraces local–regional control of disease. Thereafter, Halsted’s utilization of the techniques, which was a consolidated synthesis of the development and writings of multiple surgeons who preceded him, allowed him to achieve local and regional recurrence rates of 6% and 22%, respectively, with this en bloc approach (1,2,3). Meyer (4), in a simultaneous publication, suggested that reduction in local recurrence rates to 6% from rates of 54% to 82% acknowledged by European surgeons was a masterful contribution to the treatment of this organ. Table 20.1 compares these operations available to European surgeons during the Halstedian era, with an accompanying 3-year estimated “cure” rate for breast cancer.
In its final anatomical design, the technique of the Halsted radical procedure espoused by both Halsted and Meyer embodied the following concepts and principles:
wide excision of the skin, covering the defect with Thiersch grafts;
routine resection of both pectoral muscles;
routine axillary dissection (Levels I, II, III); and
resection of all tissues en bloc, providing wide skin and surgical margins for all margins of tumor growth.
The design and application of cytoreduction of the advanced primary mammary neoplasm has provided the medical oncologists and surgeons a vast increase in the latitude of application of less radical procedures in the therapy of carcinoma of the breast. Although modern pharmacology and breast irradiation enhance the probability of these cytoreductive events, especially with the combinations of targeted chemotherapeutic agents, the Halsted radical mastectomy is occasionally essential to achieve local–regional control of the breast, axilla, and chest wall. Quite simply put, these adjuvant therapies were the major advances that allowed less radical procedures to achieve enhanced local–regional control and survival without application of the Halsted or modified radical techniques.
Table 20.1 Chronology of the Mastectomy for Treatment of Breast Cancer with Expectant (Average) 3-Year “Cure Rates” | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 20.2 acknowledges current indications for the use of the Halsted radical mastectomy for patients presenting with advanced local–regional disease.
Major transitions from the Halstedian approach for breast cancer therapy have occurred as a result of the equivalent local–regional control that is evident with alternative breast conservation principles (19).
Unequivocally, with the increasing application of appropriate breast conservation surgery in eligible patients has been a redefinition for indications of the Halsted procedure.
Included in Table 20.2 is treatment of local recurrence following the conservation approach in the presence and/or absence of regional disease. In this circumstance, the radical mastectomy may be indicated when disease invades the pectoralis major muscle. Surgical dogma would suggest that this represents a “salvage mastectomy” necessary for large, bulky recurrences, especially for posterior lesions that recur with fascial or muscle fixation. In contemporary practice of surgical oncology, the medical or surgical oncologist should expect this event to occur in less than 2% to 5% of presenting patients following conservation approaches.
Furthermore, the radical surgical procedure may be the only available technique for local–regional control of locally advanced (fixed, ulcerated) disease, especially in patients who have received prior therapy with total breast irradiation and/or multimodal cytotoxic therapy (20,21,22).
There is no current indication for an extended radical mastectomy held in the contemporary practice of breast surgery. However, for long durations, there were proponents of the extended procedure as an advantage over the Halsted radical procedure with its principal advantage for lesions of the medial upper or lower quadrants in which the radical procedure, together with internal mammary nodal dissection,
enhanced survival (23,24,25,26). In most North American, South American, and European clinics, the extended procedure has been fully abandoned.
Table 20.2 Relative Indications for the Halsted Radical Mastectomya
Advanced locoregional disease with fixation to pectoralis major muscle (T2, T3, T4a-c; stages IIIA, IIIB, IIIC), when refractory to induction chemotherapy and irradiation
Advanced locoregional disease with skin ulceration (T4b; stage IIIb) unresponsive to radiochemotherapy
Recurrent advanced, locoregional disease (T2, T3, T4) after partial (segmental) mastectomy with tumor fixation to pectoralis major muscle (“salvage” mastectomy)
For completion of the radical procedure with locoregional recurrence after modified/segmental mastectomy when tumor invades chest wall and is refractory to cytoinduction chemotherapy
High-lying advanced peripheral lesions near clavicle/sternum with tumor fixation to muscle (stages IIA, IIB; stages IIIA, IIIB)
aAll presentations of advanced locoregional disease should receive induction cytotoxic drug therapy, radiotherapy, or both before radical mastectomy. Staging to rule out systemic disease should precede induction therapy.
From Bland and Copeland (18) with permission.
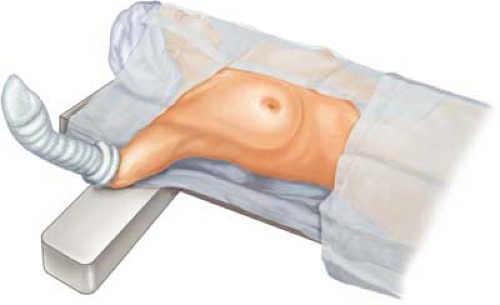
Following induction of general endotracheal anesthesia, the patient is carefully positioned with a cooperative anesthesiologist and surgeon to ensure that the patient is positioned supine on the operating table near its margin.
The ipsilateral and involved arm should have careful inspection such that the shoulder contour overlies the placement of a padded arm board and allows the ipsilateral hemithorax and shoulder to be slightly elevated on a sheet roll. This latter maneuver ensures avoidance of subluxation and abduction of the shoulder with potential stretch of the brachial plexus. Such stretch injury to the brachial plexus may initiate motor denervation (transient or prolonged) of the shoulder and arm. This complication is best avoided by padding the arm board to allow elevation of the forearm and hand, as well as providing a relaxed anatomical position (Fig. 20.1).
The operator should confirm that the ipsilateral arm and shoulder have free mobility for adduction across the chest wall; moreover, the elbow should be easily flexed and extended without undue tension (Fig. 20.2).
Prior to commencement of the surgical incision, the ipsilateral arm is placed in a relaxed, extended position on the armboard to allow proper planning of the incision. Incisions are made on the basis of guidelines discussed in this chapter. The two most common incisions employed for the Halsted radical mastectomy are the classical Orr and Stewart incisions and are illustrated in Figures 19.10 through 19.16 in chapter 19 (Modified Radical Mastectomy). Planned tissue dissections and flap elevations may be completed with electrocautery, cold scalpel, or aluminum garnet (Nd:YAG) laser scalpel (27).
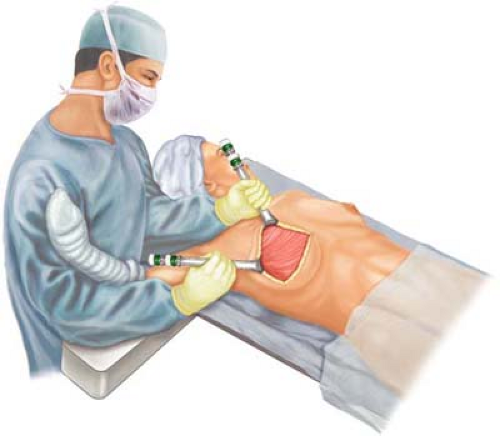
As reflected in Table 20.2, relative indications for the Halsted radical mastectomy must be applied and have decreasing usage in American surgery due to the increasing application of neoadjuvant chemotherapy with tumor cytoreduction. However, tumors that were T2, T3, or T4 with gross fixation (involvement) of the skin overlying the pectoralis major muscle (Fig. 20.3, inset) and for peripheral high-lying lesions that are fixed near the clavicle to the pectoralis major and are not otherwise candidates for radiation therapy would be considered for the procedure. Furthermore, stages IIIa, IIIb, and IIIc would be candidates for the Halsted radical mastectomy when these individuals were refractory to induction chemotherapy and/or irradiation. It is rare in conventional medical oncology to present with advanced local–regional disease and skin ulceration (T4b; stage IIIb) with tumors that are unresponsive to chemotherapy as formally noted in the medical literature. More commonly seen today is advanced local–regional disease in which there has been evident tumor fixation to the pectoralis major following segmental mastectomy and whole breast irradiation. Fixation to the pectoralis major muscle in this circumstance should have planned partial or total resection of the pectoralis major, even when radiographic evidence (mammogram, MRI [magnetic resonance imaging]) confirms evidence of cytoreduction.
It is the typical exposure of the craniolateral-most aspect of the wound that allows the identification and exposure of the humeral insertion of the pectoralis major muscle with continuation of the dissection in a central superiomedial direction with muscular elevation to allow exposure of the pectoralis minor. The insertion of the pectoralis major on the humerus is transected and rotated medially. This muscle has its origin on ribs 1 to 6 near the sternocostal junction. The surgeon must thereafter be aware of the anatomical
position of the cephalic vein and its relationship to the deltopectoral triangle. Thereafter the dissection commences medially with resection of the pectoralis major at its craniad clavicular attachments. This maneuver allows the surgeon direct exposure to the axilla. Thereafter, the tendonious portion of the pectoralis minor may be identified, encircled digitally, and stripped back to its insertion on the coracoid process of the scapula (Fig. 20.4). The pectoralis minor is likewise digitally elevated from the axilla with careful elevation from the axilla taking care to avoid injury to the axillary vein. Division and ligature of perforating muscular branches from the thoracoacromial artery and vein must be ensured. The medial (anterior thoracic) pectoral nerve commonly penetrates the pectoralis minor prior to innervation of the pectoralis major, and it should be ligated and divided on its posterior surface of origin from the medial cord of the brachial plexus.
position of the cephalic vein and its relationship to the deltopectoral triangle. Thereafter the dissection commences medially with resection of the pectoralis major at its craniad clavicular attachments. This maneuver allows the surgeon direct exposure to the axilla. Thereafter, the tendonious portion of the pectoralis minor may be identified, encircled digitally, and stripped back to its insertion on the coracoid process of the scapula (Fig. 20.4). The pectoralis minor is likewise digitally elevated from the axilla with careful elevation from the axilla taking care to avoid injury to the axillary vein. Division and ligature of perforating muscular branches from the thoracoacromial artery and vein must be ensured. The medial (anterior thoracic) pectoral nerve commonly penetrates the pectoralis minor prior to innervation of the pectoralis major, and it should be ligated and divided on its posterior surface of origin from the medial cord of the brachial plexus.
The surgeon thereafter commences medial rotation and resection of the pectoralis major and minor from the chest wall en bloc. As the medial resection of the pectoralis major continues, the lateral (anterior thoracic) pectoral nerve




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree