Autologous Reconstruction: Microvascular TRAM and DIEP Flap
David W. Chang
Geoffrey L. Robb
Since it was first described in 1979, the free transverse rectus abdominis myocutaneous (TRAM) flap has become one of the most popular and reliable methods of microsurgical breast reconstruction. Over the years, the free TRAM flap has evolved to the muscle-sparing (MS) TRAM flap and the deep inferior epigastric perforator (DIEP) flap to minimize donor site morbidity by harvesting less muscle and less anterior rectus fascia. Each flap transfers the same lower abdominal skin and subcutaneous tissue to provide an aesthetically pleasing breast reconstruction.
Indications
A free TRAM/DIEP flap can be used for breast reconstruction in the overwhelming majority of mastectomy patients. A possible candidate for breast reconstruction with a free TRAM/DIEP flap is a healthy patient with moderate amounts of abdominal skin laxity and fat and a minimal to moderate volume requirement for breast reconstruction. The patient must be willing to undergo the long, complex procedure and accept the possibility of a prolonged postoperative recovery. She must also understand and accept that she will have an additional scar in the abdomen and potential donor site morbidities.
Contraindications
A patient is not a candidate for a free TRAM/DIEP flap if she
is unwilling to accept an additional donor site scar and potential donor site morbidities;
is unwilling to undergo the long, complex procedure with a prolonged postoperative recovery;
has an abdominal donor site that cannot be closed primarily because she is too thin or has a potbelly habitus;
has had a previous TRAM flap or abdominoplasty;
has had a previous abdominal surgery in which the deep inferior epigastric vessels were divided or damaged; or
has significant medical comorbidities that make her a poor surgical candidate.
High-Risk Patients
Smokers
Because of the free TRAM flap’s excellent blood supply, a patient’s history of smoking, by itself, is not an absolute contraindication for use of a free TRAM flap. In our experience, free TRAM flap breast reconstruction in tobacco smokers is not associated with a significant increase in the rates of vessel thrombosis, flap loss, or fat necrosis compared with rates in nonsmokers. However, smokers are at significantly higher risk for mastectomy skin flap necrosis, abdominal flap necrosis, and abdominal hernia compared with nonsmokers. These smoking-related complications can be significantly reduced when the patient stops smoking at least 4 weeks before surgery.
However, for free MS-TRAM flaps and DIEP flaps, where only selected few perforators are included and the perfusion of the flap is not as robust as in free TRAM flaps, we recommend a more cautious approach. For smokers, a safer approach is to optimize the perfusion to the flap by incorporating multiple perforators, thus minimizing fat necrosis and other flap-related complications. Therefore, a free TRAM or free MS-TRAM flap is recommended for smokers, and it is safer to avoid a DIEP flap.
Obese Patients
The decision about whether to use a free TRAM flap for breast reconstruction in an obese patient should be individualized. In our experience, obese patients have significantly higher flap and donor site complications than normal-weight patients. Specifically, compared with normal-weight patients, obese patients have significantly higher rates of total flap loss, flap seroma, mastectomy skin flap necrosis, abdominal hernia, donor site infection, and donor site seroma. In fact, there appears to be an almost linear relationship between complications of all kinds and body weight. Thus, for morbidly obese patients (body mass index of ≥40), TRAM flap breast reconstruction probably should be avoided, if possible. For patients who are obese but not morbidly obese (body mass index of ≥30 but <40), free TRAM flap reconstruction may be considered if a patient is in otherwise good health and is well informed about the increased risk of complications. An obese patient undergoing delayed breast reconstruction should be encouraged to reduce her risk by losing weight prior to the surgery.
As in smokers, the use of DIEP flaps where only selected few perforators are included and the perfusion of the flap is not as robust as in free TRAM flaps, a more cautious approach is recommended for obese patients. A safer approach for these patients is to optimize the perfusion to the flap by incorporating multiple perforators with use of a free TRAM or free MS-TRAM flap, thus minimizing fat necrosis and other flap-related complications.
Previous Abdominal Suction-Assisted Lipectomy
There is debate about whether a free TRAM flap can be reliably used following abdominal liposuction. The obvious concern is that the perforating vessels to the flap and the microvasculature to the flap may have been damaged by the prior suction-assisted lipectomy (SAL) procedure, which could compromise the viability of the flap. However, a few cases of free TRAM flap breast reconstruction following SAL of the abdomen have been reported. When this is being considered, preoperative Doppler ultrasonography can be used to confirm the presence and the patency of the perforating vessels of the abdominal wall. In addition, the surgeon should consider incorporating a maximum number of perforators into the flap to render it more robust for transfer.
Patient Evaluation
Free flap procedures impose major surgical stress on the patient. Two simultaneous operative sites cause considerable fluid loss, and patients tend to become hypothermic because of the lengthy nature of these procedures. Thus, candidates for free flap reconstruction must have their cardiac, pulmonary, and renal statuses carefully evaluated preoperatively.
Patients must be advised to abstain from smoking preoperatively for at least 4 weeks prior to surgery to reduce the risks of anesthetic complications and wound healing problems. Avoiding aspirin-containing products for 2 weeks before surgery is also important so that the baseline coagulation status is normal.
The patient’s abdomen should be evaluated to make sure she is a good candidate for a TRAM flap. In particular, the abdomen should be examined for scarring. If there are scars, their location, length, duration, and cause must be considered to determine whether a free TRAM flap can be performed safely. The abdomen should be examined with the patient in a supine position, with the knees flexed to ensure that the abdomen can be closed primarily after harvest of the TRAM flap. In addition, the integrity of the abdominal wall must be examined for the presence of hernias and for potbelly habitus.
Once it is determined that the patient is a good candidate for a free TRAM flap, the design of the flap is marked with the patient standing. The inframammary folds are marked bilaterally. The TRAM flap is designed in the lower abdomen with a transverse skin flap. The upper marking is usually just at or above the umbilicus, and the lower marking is just above the pubis, generally following the natural skin fold there. The design of the flap is then tapered to the anterior superior iliac spine so that closure of the donor site will not result in a dog-ear (Fig. 29.1).
Adjuvant Therapy
Advances in adjuvant therapies for patients with breast cancer have significantly reduced the disease’s recurrence rate and associated mortality rate. Adjuvant therapies may include systemic therapy (including cytotoxic, endocrine, or biologic modulators) and/or localized treatment (such as radiation therapy). When considering breast reconstruction for patients who need adjuvant therapy, surgeons must take into account the potential effects of breast reconstruction on adjuvant therapy, and vice versa. The safety, efficacy, and timing of breast reconstruction in patients who require adjuvant therapy must be evaluated to ensure that reconstruction does not delay adjuvant therapy or negatively affect disease-free interval or overall survival. Thus, the impact of adjuvant treatment on the approach and overall outcome of breast reconstruction merits clarification.
Chemotherapy
Many studies have shown that neoadjuvant chemotherapy followed by mastectomy and immediate breast reconstruction is safe and viable, does not delay other adjuvant treatment, and can be used to identify patients who do not respond to chemotherapy, which enables oncologists to modify postsurgical treatment. Generally, neoadjuvant chemotherapy is not a contraindication to immediate breast reconstruction and does not increase the complication rate or significantly delay further adjuvant therapy. At our institution, we recommend delaying reconstruction for 3 to 4 weeks following neoadjuvant chemotherapy to allow the immunosuppressive effects of the chemotherapy to resolve.
Although whether delaying adjuvant chemotherapy affects cancer-related outcomes is not yet definitively known, most oncologists prefer to initiate therapy 4 to 6 weeks after mastectomy or breast-conservation surgery because of concerns that longer periods may increase recurrence or diminish survival. Immediate breast reconstruction may increase the risk of complications as a result of the additional surgical procedures performed, but it does not seem to delay adjuvant chemotherapy or affect overall survival and recurrence rates.
Hormone Therapy
Despite its benefits, 1% to 2% of patients on tamoxifen may experience thromboembolic events, such as deep vein thromboses, pulmonary embolisms, and cerebrovascular thrombi. Because tamoxifen presents a theoretical risk of thrombosis, it may be appropriate to have the patient stop tamoxifen therapy 10 to 14 days before undergoing free flap reconstruction and restart the therapy after breast reconstruction. However, we recommend consulting with the patient’s medical and surgical oncologists to confirm that tamoxifen therapy can be stopped safely without negatively affecting the patient’s cancer treatment.
Biological Therapy
Trastuzumab, a humanized monoclonal antibody directed against the human epidermal growth receptor, has been shown to significantly improve survival rates in metastatic breast cancer patients when used alone or in combination with chemotherapy. Patients who receive trastuzumab alone or in combination with other chemotherapy may experience neutropenia and an increased incidence of infections. An increase in the incidence of thrombotic events has also been reported. Because of these potential complications, we recommend that patients complete trastuzumab therapy and undergo immune status evaluation before undergoing breast reconstruction. As always, we recommend consulting with the patient’s medical and surgical oncologists before making a final decision.
Radiotherapy
Given reports of the increased risk of capsular contracture associated with radiotherapy and implant reconstruction and the need for removal or reoperation despite reported acceptable cosmetic results, we recommend autologous tissue-based reconstruction instead of implant reconstruction in patients who have received or will receive radiotherapy.
For some surgeons, immediate reconstruction with autologous tissue remains the preferred approach in patients who require adjuvant radiotherapy. However, many feel that
irradiating a reconstructed breast may diminish the aesthetic outcome and thus advocate delaying reconstruction in patients who require adjuvant radiotherapy. Also, delaying reconstruction until after radiotherapy decreases the risk of fat necrosis, volume loss, and the need for additional flaps. Furthermore, immediate reconstruction may cause technical problems when designing the radiation fields necessary to deliver adjuvant radiotherapy.
irradiating a reconstructed breast may diminish the aesthetic outcome and thus advocate delaying reconstruction in patients who require adjuvant radiotherapy. Also, delaying reconstruction until after radiotherapy decreases the risk of fat necrosis, volume loss, and the need for additional flaps. Furthermore, immediate reconstruction may cause technical problems when designing the radiation fields necessary to deliver adjuvant radiotherapy.
Relevant Anatomy
Rectus Abdominis Muscle
The rectus abdominis muscles are a pair of long, straight muscles that flex the spine and tighten the intraabdominal wall. They arise from the symphysis pubis and the pubic crest and insert on the linea alba and at the fifth, sixth, and seventh costal cartilages. Each rectus abdominis muscle is subdivided by two to five tendinous inscriptions, with the most caudal one at the level of the umbilicus. The tendinous inscriptions are adherent to the overlying anterior rectus sheath but not to the posterior sheath. The inscriptions do not usually extend completely through the muscle and may pass only halfway across it.
Rectus Sheath
The rectus abdominis muscles are enclosed by a thick sheath, except for the posterior part below the arcuate line. The rectus sheath is attached to the anterior aspect of the muscles by fusion to the tendinous inscriptions. The aponeurotic extensions of the muscles of the intraabdominal wall merge to form the anterior portion of the rectus sheath fascia.
An important transition is in the posterior sheath at the arcuate line (semicircular line, or arc of Douglas). The arcuate line is generally located halfway between the umbilicus and symphysis pubis, although this is variable. The arcuate line marks the transition point where the internal oblique aponeurosis ceases to split and the aponeuroses of all three muscles pass ventral to the rectus abdominis. The transversalis fascia is the only layer present below the arcuate line and is thus a region of weakness and potential herniation after flap dissection.
The linea alba represents the decussation of the fused aponeuroses in the midline. The linea alba is wider in the region of the xiphoid process and narrows to a fine line below the umbilicus. The lateral border of the rectus sheath is often discernable externally and is referred to as the linea semilunaris.
Blood Supply
The rectus abdominis muscle has two vascular pedicles, one composed of the deep superior epigastric artery (DSEA) and the other of the deep inferior epigastric artery (DIEA) (Fig. 29.2). The DSEA and DIEA pedicles arborize as they approach each other under the surface of the rectus abdominis. These two systems connect above the umbilicus through a system of small-caliber vessels that Taylor and Palmer refer to as “choke” vessels.
The DSEA arises from the internal mammary artery at the level of the sixth intercostal space. It generally has two venae comitantes. There is a small branch of the DSEA that courses along the costal margin to join the intercostal artery lateral to the rectus sheath.
The DIEA usually originates 1 cm above the inguinal ligament from the medial aspect of the external iliac artery, directly opposite the deep circumflex iliac artery. The main DIEA pierces the transversalis fascia and enters the rectus sheath just below the arcuate line. It then ascends obliquely and medially between the rectus abdominis muscle and the posterior wall of the sheath. Generally, the DIEA divides into two or three large branches below the level of the umbilicus. Through cadaveric studies, the degree
of arborization of the DIEA has been classified into three different types. In type 1, the DIEA does not divide and remains as a single vessel as it courses under the surface of the rectus abdominis (29%). Type 2 refers to a DIEA that divides into two dominant branches (57%). The type 3 pattern is a trifurcation of the DIEA (14%).
of arborization of the DIEA has been classified into three different types. In type 1, the DIEA does not divide and remains as a single vessel as it courses under the surface of the rectus abdominis (29%). Type 2 refers to a DIEA that divides into two dominant branches (57%). The type 3 pattern is a trifurcation of the DIEA (14%).
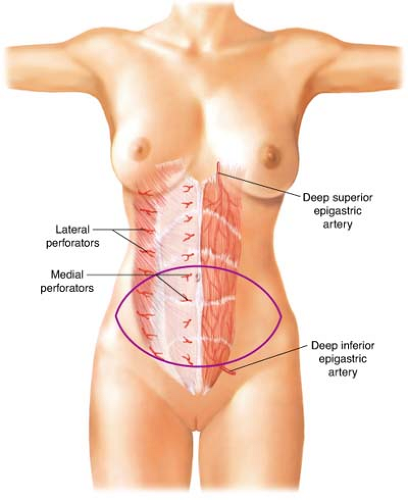 Figure 29.2 The rectus abdominis muscle has two vascular pedicles, one composed of the deep superior epigastric artery and the other of the deep inferior epigastric artery. |
The DIEA has two venae comitantes, which usually join to form a single vein prior to their junction with the external iliac vein. In the study by Boyd et al., the deep inferior epigastric veins (DIEVs) entered the external iliac vein as a single trunk in 68% of cases and as a double trunk in 32%.
Perforators
The deep arteries supply the TRAM flap’s overlying abdominal skin by a system of perforators. These vessels are terminal branches of the DIEA and DIEV. Cadaveric studies by Taylor and Palmer demonstrated a rich connection between the DIEA system and the abdominal wall skin. Many perforating arteries emerge through the anterior rectus sheath, but the highest concentration is in the periumbilical area. The fewest number of perforators is found in the suprapubic area. The branches of the periumbilical perforators have the appearance of the radiating spokes of a wheel whose hub is located at the umbilicus. Thus, incorporation of the periumbilical perforators permits the harvesting of a skin flap with virtually any orientation from the midline.
The perforators communicate with the other regional superficial vessels through a system of choke vessels that link these territories and allow the design of large skin islands based on the DIEA. The dominant connections between these systems occur within the subdermal plexus.
TRAM Flap
The blood supply to the TRAM flap is a two-tiered arrangement of muscular and subcutaneous networks. The superior and inferior epigastric artery systems form a deep longitudinal blood supply that is linked to the lower six intercostal vessels and the ascending branch of the deep circumflex iliac artery within the muscles of the abdominal wall. The DIEA is the dominant vessel of the rectus abdominis muscle and the TRAM flap. Injection of this vessel with dye will stain the abdominal wall as high as midway between the umbilicus and xiphoid process, whereas injection of the DSEA with dye rarely results in staining below the umbilicus. The subcutaneous network consists of branches of the superficial epigastric artery, superficial circumflex iliac artery, external iliac artery, superficial superior epigastric artery, and the intercostal arteries. The subcutaneous and deep systems are connected by perforators that traverse the rectus abdominis muscles.
Extensive studies of the venous circulation of the TRAM flap have revealed both superficial and deep systems. The veins of the superficial system are above Scarpa fascia and communicate extensively across the midline. The superficial veins drain into the deep venous system by way of the veins accompanying the musculocutaneous arterial perforators. Valves located in the connecting veins regulate the direction of blood flow from the superficial toward the deep system.
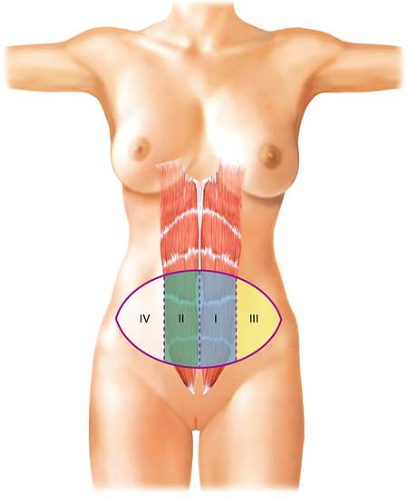
A TRAM flap incorporates skin from the entire lower abdomen. Four different skin zones can be included in a TRAM flap (Fig. 29.3). Zone 1 refers to the skin overlying each lateral rectus abdominis muscle. Zone 2 denotes skin of the contralateral lower abdomen overlying the opposite rectus abdominis muscle. The skin territory on each side of the abdomen lateral to the linea semilunaris is referred to as zone 3, and the skin lateral to the opposite linea semilunaris is zone 4. The blood supply to zone 4 is the most tenuous.
Innervations
The rectus abdominis muscles are innervated in a segmental fashion from the lower six intercostal nerves, derived from T7–T12, which traverse the plane between the transversus abdominis and the internal oblique muscles. These mixed motor and sensory nerves provide innervation to the rectus abdominis muscles and sensory supply to the overlying skin. The intercostal nerves enter the mid-portion of the rectus muscles at the posterior surface.