Intraoperative Ultrasound-Guided Excision of Nonpalpable Lesions
V. Suzanne Klimberg
Introduction
It has been little more than three decades since mammograms were first employed. Mammograms have contributed greatly to the decreased size at which breast cancer can be diagnosed. Initially nonpalpable lesions were removed by the surgeon’s best estimate of where the mass or calcification was located in the breast from triangulation of the mammogram views. Large resections were usually needed often for benign lesions, targeted entities more frequently missed, and reoperations more often required when cancer was diagnosed. Needle localization breast biopsy (NLBB) (see Chapter 7) became the new standard in the 1980s for diagnosis and removal of nonpalpable lesions. Miss rates remain in the 5% to 9% range and vasovagal episodes seen in 10% to 20% percent of patients. Today, the consensus is that image-guided needle core biopsy prior to surgery is the standard of care and can determine whether open biopsy can be avoided (in up to 80% of cases) or whether cancer is present and more than just excision is needed.
In the last decade, surgeons have become very facile with ultrasound (US). As more than half of nonpalpable masses diagnosed on mammography can be seen with US, intraoperative-guided excisional biopsy became in vogue for lesions that needed complete removal. Unfortunately, this left an increasing number of patients diagnosed on mammography with calcifications. Stereotactic needle core biopsy was developed in the 1990s in order to minimally invasively biopsy microcalcifications and non–US-visualized masses. If after stereotactic biopsy further open excision was required, NLBB of the stereotactically placed clip has remained the standard to remove such lesions. Recently, hematoma-directed ultrasound-guided (HUG) excision has been described. This method utilizes intraoperative US to locate the hematoma left behind after image-guided core biopsy (mass or calcifications) and intraoperatively guide the excisional biopsy by line of sight.
Indications for US-guided excisional breast biopsy (EBB) include those lesions described as indeterminate or suspicious on core biopsy including but not exclusive of atypical lobular or ductal hyperplasia, incompletely excised papillomas and papillomas associated with atypia whether completely excised or not, lobular carcinoma in situ, radial scar, and nonconcordance of imaging with the pathology. Occasionally, patients will present with a nonpalpable lesion that is almost certainly benign but is greater than 2 cm in size or is growing, painful, or recurring (e.g., a previously aspirated cyst) or simply because of patient preference or fear of needles.
Contraindications to intraoperative US-guided biopsy is simply poor or nonvisualization of the lesion after core biopsy. This may occur in patients if the image-guided core biopsy has been longer than 5 weeks. By this time significant resorption of the biopsy cavity hematoma would have occurred. Hematoma-directed ultrasound-guided biopsy may also not be useful if there is a massive hematoma after image-guided core biopsy, making the localization relatively nonspecific.
Preoperative Core Biopsy and Diagnosis
Although some advocate the use of fine needle aspiration, a definitive diagnosis via core needle biopsy before performing EBB is preferred. All outside pathology should require rereview within the institution performing the EBB. A preoperative benign diagnosis (e.g., fibroadenoma) will permit some patients to forgo EBB. A preoperative diagnosis of cancer allows for a single definitive cancer operation in most cases compared with only 25% to 60% of patients in whom NLBB is used for diagnosis.
Ultrasound
A high-quality US is needed for intraoperative use and it should be tested prior to surgery and the settings appropriate to the patient’s breast and transducer locked in. For intraoperative use, the small 15-MHz probe is ideal. The US should be placed on the opposite side of the table from where the surgeon will be standing for ease of visualization during the procedure. The US should be covered with a sterile clear drape such that the operator can adjust the machine with sterile technique during the procedure. Likewise, a sterile drape will be needed to cover the US transducer (Fig. 8.1).
Relevant Anatomy
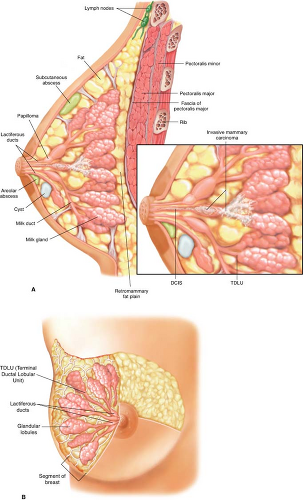
Figure 8.2A demonstrates the intraductal nature of cysts, papilloma, atypia, and ductal carcinoma in situ (DCIS), with most cancers thought to arise from the terminal ductal
lobular unit of the breast, and Figure 8.2B demonstrates the radial nature of the segments of the breast.
lobular unit of the breast, and Figure 8.2B demonstrates the radial nature of the segments of the breast.
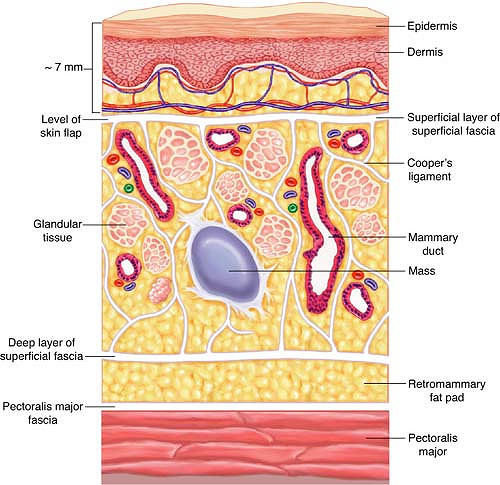
Figure 8.3 demonstrates the important layers of the breast in cross-section. Retention of the subcutaneous layer just below the skin significantly contributes to the cosmesis after EBB. Likewise, preservation of the integrity of posteriorly located fascia covering the pectoralis major prevents the formation of tense seromas.
Surgical Technique
Anesthesia
Excision of breast lesions can be performed under local, with or without sedative, or general anesthesia, although some reports have suggested better margin clearance under general anesthesia. With a very cooperative patient, local anesthesia with a mixture of short- and long-acting anesthetic with epinephrine and mild sedation is easily doable.
Positioning
The patient is placed supine or in a lawn chair position, with the arm on the affected breast extended at a right angle such that the operator and the assistant can stand on either side of the arm board (Fig. 8.4). The patient should be at the very edge of the table so that the surgeon can stand in close proximity to the patient and not have to unduly lean over the table to operate (spares back strain). The US monitor is positioned on the opposite side of the table in direct line of sight of the surgeon. The arm does not need to be draped into the field but should be place on sufficient padding to relieve any pressure on the radial nerve at the elbow. After sterile prep, a biodrape can be used to retract and stabilize a large pendulous breast in an optimal position for EBB.
Intraoperative Localization
A 7.5-, 10-, or 15-MHz US transducer can be used to locate a nonpalpable mass or the residual hematoma from an image-guided biopsy of a nonpalpable mass or calcifications (Fig. 8.5A–D). A purple pen is used to outline the mass located by the US probe and determine the best position for the incision.