Radiation Therapy and Breast Reconstruction: A Critical Review of the Literature
Steven J. Kronowitz, M.D.
Geoffrey L. Robb, M.D.
Houston, Texas
From the Department of Plastic Surgery, University of Texas M. D. Anderson Cancer Center.
Received for publication February 25, 2008; accepted January 27, 2009.
Copyright ©2009 by the American Society of Plastic Surgeons
DOI: 10.1097/PRS.0b013e3181aee987
Disclosure: The authors have no financial interests to declare in relation to the content of this article.
Background: The optimal timing and technique of breast reconstruction in patients who may require postmastectomy radiation therapy are controversial. To help surgeons make the best decisions, the authors reviewed the recent literature on this topic.
Methods: The authors searched the MEDLINE database for studies of radiation therapy and breast reconstruction with most patients treated after 1985 and mean follow-up of more than 1 year. Forty-nine articles were reviewed.
Results: Even with the latest prosthetic materials and modern radiation delivery techniques, the complication rate for implant-based breast reconstruction in patients undergoing postmastectomy radiation therapy is greater than 40 percent, and the extrusion rate is 15 percent. Modified sequencing of two-stage implant reconstruction, such that the expander is exchanged for the permanent implant before postmastectomy radiation therapy, results in higher rates of capsular contracture and is not generally feasible after neoadjuvant chemotherapy. Current evidence suggests that postmastectomy radiation therapy also adversely affects autologous tissue reconstruction. Even with modern radiation delivery techniques, immediate implant-based or autologous tissue breast reconstruction can distort the chest wall and limit the ability to treat the targeted tissues without excessive exposure of the heart and lungs. In patients for whom postmastectomy radiation therapy appears likely but may not be required, “delayed-immediate reconstruction,” in which tissue expanders are placed at mastectomy, avoids the difficulties associated with radiation delivery after immediate reconstruction and preserves the opportunity for the aesthetic benefits of skin-sparing mastectomy.
Conclusions: In patients who will receive or have already received postmastectomy radiation therapy, the optimal approach is delayed autologous tissue reconstruction after postmastectomy radiation therapy. If postmastectomy radiation therapy appears likely but may not be required, delayed-immediate reconstruction may be considered. (Plast. Reconstr. Surg. 124: 395, 2009.)
Postmastectomy radiation therapy can improve survival and locoregional control in patients with invasive breast cancer. The optimal timing and technique of breast reconstruction in patients requiring postmastectomy radiation therapy are controversial. The purpose of this review was to examine the most recent literature on breast reconstruction in patients receiving postmastectomy radiation therapy to help plastic surgeons make the best treatment decisions.
Patients and Methods
To find articles for review, we performed a search of the MEDLINE database for studies of radiation therapy and breast reconstruction. We then read the reference lists of the identified articles to find additional articles for review. Studies were included if most patients were treated after 1985 and the mean follow-up period was more than 1 year. Forty-nine articles were reviewed.
Table 1. Locoregional and Survival Outcomes in the Danish and British Columbia Randomized Trials of Postmastectomy Radiotherapy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Indications for Postmastectomy Radiation Therapy: Consensus and Controversies
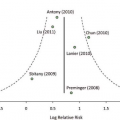
Postmastectomy radiation therapy improves the outcomes of breast cancer patients whose risk of locoregional recurrence is greater than 25 to 30 percent.1–3 There is consensus that patients with T3 or T4 tumors or four or more positive axillary lymph nodes have this risk. The American Society for Therapeutic Radiology and Oncology,4 the American Society of Clinical Oncology,5 and Health Canada’s Canadian Breast Cancer Initiative6 recommend postmastectomy radiation therapy for breast cancer patients with advanced disease (T3 or T4 tumors or at least four positive axillary nodes).
However, the role of postmastectomy radiation therapy in the treatment of patients with T1 or T2 tumors and one to three positive axillary nodes is controversial. Although the Danish and British Columbia prospective trials reported a risk of locoregional recurrence ranging from 20 to 30 percent,1,7 postmastectomy radiation therapy reduced this risk by two-thirds (Table 1).3 Most contemporary retrospective analyses of postmastectomy locoregional recurrence in women with T1 or T2 breast cancer with one to three positive axillary lymph nodes treated with systemic chemotherapy but without postmastectomy radiation therapy report an approximate 10-year locoregional recurrence risk of 15 percent. Woodward and colleagues reported a reduction in locoregional recurrence rate from 13 percent without postmastectomy radiation therapy to 3 percent with postmastectomy radiation therapy in patients with one to three positive nodes.8 The heterogeneity of the reported risk for locoregional recurrence in patients with T1 or T2 tumors and one to three positive nodes indicates that more prospective studies are needed to establish how much the addition of postmastectomy radiation therapy to mastectomy and systemic chemotherapy reduces locoregional recurrence risk and improves survival.
A study at the University of Texas M. D. Anderson Cancer Center found that extranodal extension of at least 2 mm, fewer than 10 nodes excised, and tumor size greater than 4 cm were independent predictors of locoregional recurrence, even in patients with one to three positive nodes.9 Another study, of patients with T1 and T2 tumors with one to three positive nodes, showed that age younger than 45 years, more than 25 percent of excised nodes positive, medial tumor location, and estrogen receptor-negative status were significantly associated with locoregional recurrence risk greater than 20 percent,10 which may be a reasonable indication for postmastectomy radiation therapy.
Because of the downstaging that often occurs after neoadjuvant chemotherapy, both clinical and pathologic tumor stage must be considered in determining the need for postmastectomy radiation therapy.11 Studies indicate that patients with T3 or T4 tumors or clinical stage III disease at diagnosis clearly benefit from postmastectomy radiation therapy after neoadjuvant chemotherapy and definitive surgery, even if they have a complete response to the chemotherapy.12,13 It is unclear whether patients with T1 or T2 tumors at diagnosis, regardless of the response to neoadjuvant chemotherapy, should also receive postmastectomy radiation therapy. In the future, the need for radiation therapy may be determined solely on the basis of microarray gene analysis of core biopsy specimens obtained before surgery; permanent pathology results and downstaging resulting from
neoadjuvant chemotherapy may no longer influence treatment.
neoadjuvant chemotherapy may no longer influence treatment.
Design of Postmastectomy Radiation Therapy: Consensus, Controversies, and the Potential for Lower Dose Regimens
The chest wall is the most common site of locoregional recurrence after postmastectomy radiation therapy; therefore, the chest wall should be treated in all patients undergoing postmastectomy radiation therapy.13,14 In patients with T3 to T4 primary disease or four or more positive lymph nodes who undergo standard level I to II axillary dissection, level III (the supraclavicular field) is at high risk for locoregional recurrence and should be irradiated.13,14 For patients with stage II breast cancer with one to three positive lymph nodes, the recurrence rate in level III is 5 percent or less,13–15 and many radiation oncologists believe that the benefits of postmastectomy radiation therapy are outweighed by its risks (lymphedema rates of 4 to 11 percent after postmastectomy radiation therapy and upward of 40 percent after axillary dissection and postmastectomy radiation therapy16–19).
Treatment of the internal mammary nodes is controversial. The Danish and British Columbia trials,1–3,7 which included the internal mammary nodes within the radiation treatment fields, showed a significant improvement in overall survival in patients with one to three positive axillary nodes.3 Despite these results, many radiation oncologists believe that internal mammary failures are rare and that a benefit is probably not provided to most patients with one to three involved axillary nodes. However, this “rarity” in patients with one to three positive axillary nodes may result from our inability to detect these recurrences at the first site of failure, as in women with locally advanced breast cancer who have a high rate of internal mammary chain failure (20 to 50 percent).20–22 Although the specific criteria by which to irradiate the internal mammary nodes are currently being investigated by the European Organization for Research and Treatment of Cancer 22922 trial, irradiation may have value in patients with locally advanced disease, patients with inner or central tumors with axillary involvement, and patients with early-stage disease primarily draining to the internal mammary nodal chain as evidenced by lymphoscintigraphy.
Studies from both the United Kingdom23 and Canada24 have suggested that breast cancer patients may not need as much radiation as they have been receiving. The United Kingdom and Canada groups have already shown—in more than 4000 patients with breast cancer who underwent partial mastectomy followed by radiation therapy—that a lower total dose of radiation delivered in fewer, larger fractions does not compromise either the safety or the effectiveness of radiation therapy. Currently, the United Kingdom and Canadian groups have been investigating the use of even fewer fractions of radiation and the usefulness of hypofractionation for whole-breast irradiation after mastectomy.
Implant-Based Breast Reconstruction in Patients Receiving Postmastectomy Radiation Therapy
With an increasing number of patients receiving postmastectomy radiation therapy, the decision of whether to offer implant-based or autologous tissue breast reconstruction has never been so relevant. Recent studies indicate that implant-based breast reconstruction in patients receiving postmastectomy radiation therapy is problematic.
Outcomes of Implant-Based Reconstruction with Modern Radiation Delivery Techniques
Studies evaluating the outcomes of two-stage breast reconstruction, with placement of a tissue expander followed by placement of a permanent breast implant after postmastectomy radiation therapy, consistently reveal high rates of acute and chronic complications and poor aesthetic outcomes.25 Capsular contracture that results from postmastectomy radiation therapy not only distorts the appearance of the breast but also causes chronic chest wall pain and tightness that can be crippling. Many surgeons attribute the poor outcomes with implant-based breast reconstruction to older, less precise techniques of radiation delivery. However, even with modern radiation delivery techniques, complication rates with implant-based reconstruction are high.
Ascherman and colleagues26 recently evaluated the complications and aesthetic outcomes of 104 patients who underwent two-stage implant-based reconstruction. Twenty-seven patients also underwent radiation therapy, either before mastectomy (patients who were undergoing salvage mastectomy after lumpectomy and radiation therapy) or after mastectomy. In all 27 of these patients, radiation therapy was completed before the tissue expander was exchanged for a permanent breast implant or before the expander port was removed. Despite use of the latest prosthetic materials and modern radiation delivery techniques, the overall complication rates for the irradiated and nonirradiated breasts were 40.7 percent and 16.7 percent, respectively (p ≤ 0.01). Complications that resulted in removal or replacement of the prosthesis occurred in 18.5 percent of the irradiated and only 4.2 percent of the nonirradiated breasts (p ≤ 0.025). In addition, the extrusion rate was higher for implants in irradiated breasts (14.8 percent versus 0 percent; p ≤ 0.001). Breast symmetry scores were significantly higher in the patients who did not receive radiation therapy (p < 0.01). Despite the retrospective study design, these findings are important because they represent the experience of a single surgeon who evaluated patients treated using the latest prosthetic devices with total submuscular coverage and modern radiation therapy techniques.
In another recent study,27 Benediktsson and Perbeck used applanation tonometry to prospectively evaluate rates of capsular contracture around saline-filled, textured implants in 107 patients who underwent mastectomy with immediate breast reconstruction. Twenty-four of the patients received postmastectomy radiation therapy. Radiation was delivered using a modern, three-beam technique with a combination of photons and electrons, and reconstruction was accomplished using the latest prosthetic devices. The rate of capsular contracture was significantly higher for irradiated breasts than for nonirradiated breasts (41.7 percent versus 14.5 percent; p = 0.01). The difference in contracture rates was not evident during the first 6 months but was highly significant thereafter, even 5 years later.
In 2006, Behranwala and colleagues28 published results from a series of 136 breast reconstructions with a median follow-up of 4 years: 62 reconstructions were performed with submuscular implants alone and 74 were performed with a latissimus dorsi flap plus an implant. Forty-four reconstructed breasts received postmastectomy radiation therapy. Capsule formation was detected in 14.1 percent of the nonirradiated reconstructed breasts and 38.6 percent of the irradiated reconstructed breasts. On univariate analysis, postmastectomy radiation therapy was the only variable related to capsule formation (p < 0.001). Significant differences in geometric measurements of the breasts and worse photographic assessments were seen in the group that received postmastectomy radiation therapy. Pain that persisted for 2 years or more after surgery was present in 27 percent of breasts with capsular contracture and less than 1 percent of breasts without capsular contracture.
Impact of Performing the Tissue Expander–Permanent Implant Exchange before Rather than after Radiation Therapy
In a departure from the standard approach of exchanging the tissue expander for the permanent breast implant after postmastectomy radiation therapy, Cordeiro and colleagues have been exchanging the expander for the implant before postmastectomy radiation therapy. Whereas Ascherman and colleagues26 had to remove or replace the implant in 18.5 percent of the irradiated patients who had the permanent implant placed after postmastectomy radiation therapy, Cordeiro and colleagues29 had to remove or replace the implant in only 11.1 percent of 81 patients. In addition, their technique resulted in acceptable aesthetic outcomes in most patients.
Although placing the permanent implant before postmastectomy radiation therapy should decrease the occurrence of acute wound-healing problems (i.e., wound dehiscence, infection, and implant exposure), there is no known pathophysiologic explanation for the relatively low frequency (5.9 percent) of severe (Baker classification, grade 4) capsular contracture described in the study by Cordeiro and colleagues. In fact, the literature is replete with studies showing much higher rates of severe capsular contracture—in the range of 16 to 68 percent—when the permanent breast implant is in situ during the delivery of postmastectomy radiation therapy.27,28
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree