Proper wound care has broad applications for all clinicians. Much of the future direction for enhancing wound repair focuses on key cells and growth factors, which is why possessing a strong understanding of the basic physiology of wound healing is imperative. This article first provides a thorough review of the phases of wound healing followed by a discussion on the latest wound management strategies. Wound conditions and surgical techniques are important components for optimizing wound healing and preventing complications. Special consideration has been given to the unique settings of contaminated wounds, open wounds, or avulsed tissue.
The proverb states, “time heals all wounds” and, fortunately, through the resilience of the human body, this often holds true. As medicine advances, so has understanding of the mechanisms of wound healing. Elucidation of the healing process has permitted better opportunities to promote healing while minimizing scar formation. We possess a gross understanding of the key cells and factors that manipulate healing; however, the ability to translate this knowledge into clinical use is still lacking and limits the potential to completely control and enhance the process.
Surgery inherently implies the creation of a wound and, consequently, all wounds create scar. Therefore, an important and unspoken goal in surgery is to accomplish the procedure while minimizing scar formation. Proper surgical techniques for wound closure have long been described, and through continued affirmation, these concepts have evolved into tenets rather than mere recommendations.
Wound healing is important in a wide range of scenarios, whether a patient is healing from a chronic open ulcer or healing from a planned scar revision. The ability to optimize the early care of wounds can greatly aid in the expediency of a wound healing, as well as in the final aesthetic appearance. Although there is no absolute way to care for a wound, this article describes key points that can aid in promoting improved healing while preventing future complications, such as the development of a chronic wound, a hypertrophic scar, or a visibly undesirable scar.
Wound healing phases
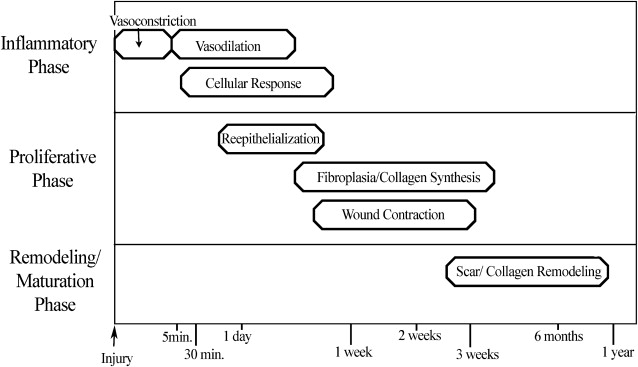
Understanding the basic stages of wound healing is imperative to best regulate the process. The physiology of wound healing involves multiple phases over time and can be organized into the following phases ( Fig. 1 ):
- •
Inflammatory
- •
Proliferative
- •
Remodeling/maturation.
Inflammatory Phase
During the initial injury, hemostasis is of primary importance, and immediate vasoconstriction occurs. Vasoconstriction is mediated by thromboxane A 2 and lasts for 5 to 10 minutes. Endothelial cell injury and exposure of collagen, fibronectin, and laminin lead to activation of the coagulation and complement cascades, which initiate the formation of a clot consisting of fibrin and aggregated platelets. Activated platelets release prostaglandins and vasoactive materials, such as serotonin, histamine, proteases, and thromboxane, which go on to activate their target cells ( Box 1 ).
Substances Released From α Granules of Platelets During Wound Healing
Platelet-derived growth factor
Basic fibroblast growth factor
Vascular endothelial growth factor
Transforming growth factor β1
Transforming growth factor α
Epidermal growth factor
Thrombospondin
Platelet thromboplastin
Coagulation factors
Serotonin
Histamine
Platelet-activating factor
Hydrolytic enzymes
Endostatin (antiangiogenic)
After initial vasoconstriction, active vasodilation occurs, likely secondary to histamine release from mast cells and circulating serotonin. Subsequently, kallikrein is activated, leading to kinin activation and endothelial cell separation, which then allows increased vascular permeability that continues for the first 48 to 72 hours.
The cellular response in the inflammatory phase lags somewhat behind the vascular changes, and it begins as fibronectin promotes the migration of neutrophils, monocytes, fibroblasts, and endothelial cells into the region of injury. Fibronectin forms cross-links with clot, which epithelial cells and fibroblasts use as a temporary matrix to proliferate in the wound. Polymorphonuclear leukocytes (granulocytes) and monocytes are among the first cells to appear after an injury. Stimulated by chemotactic factors, granulocytes appear within 6 hours of an insult, and act to clean the wound by phagocytic removal of bacteria and debris. In a noncontaminated wound, the presence of granulocytes is generally short-lived; however, in a contaminated wound, granulocytes can persist and prolong the inflammatory phase. Lengthened periods of inflammation may account for worsened scarring.
Macrophages are essential for wound healing by providing a critical regulatory function in the inflammatory phase and by transitioning a wound into a stage of repair. Attracted by platelet-derived growth factor (PDGF), macrophages are the predominant cell type in a wound by 48 to 96 hours. Macrophages release chemotactic and growth factors, such as transforming growth factor β (TGF-β), basic fibroblast growth factor (FGF), epidermal growth factor, transforming growth factor-α (TGF-α), and PDGF, that result in endothelial and fibroblast proliferation ( Table 1 ). If macrophage function is diminished, granulation tissue formation, fibroplasia, collagen production, and, subsequently, overall wound healing, are decreased. The immune response is also closely linked to wound repair, because lymphocytes produce important factors, such as TGF-β, interferons, interleukins, and tumor necrosis factor, that interact with macrophages.
| Cytokine | Abbreviation | Source | Function |
|---|---|---|---|
| Human growth hormone | GH | Pituitary gland | Fibroblast proliferation; increases collagen content and tensile strength; anabolism; stimulates IFG-1 |
| Epidermal growth factor | EGF | Platelets, bodily fluids | Epithelial cell and fibroblast proliferation and migration; activates fibroblasts; angiogenic |
| Platelet-derived growth factor | PDGF | Platelets, macrophages, fibroblasts, endothelial cells, smooth muscle cells | Mitogenic for fibroblasts and smooth muscle cells; chemoattractant for neutrophils and macrophages; angiogenic |
| Fibroblast growth factor | FGF | Macrophages, brain, pituitary gland | Proliferation and migration of vascular endothelial cells; mitogenic and chemotactic for keratinocytes and fibroblasts |
| Transforming growth factor | TGF | Platelets, fibroblasts, neutrophils, macrophages, lymphocytes | Epithelial cell and fibroblast factors proliferation |
| Insulinlike growth factor 1 | IGF-1 | Fibroblasts, liver, plasma | Fibroblast proliferation; proteoglycan and collagen synthesis |
| Tumor necrosis factor | TNF | Macrophages, mast cells, lymphocytes, other tissues and cells | Fibroblast proliferation |
| Interleukins | ILs | Macrophages, lymphocytes, other tissues and cells | Fibroblast proliferation; neutrophil chemotaxis |
| Interferons | IFNs | Fibroblasts, lymphocytes | Inhibition of fibroblast proliferation and collagen synthesis |
| Keratinocyte growth factors | KGFs | Fibroblasts | Epithelial cell proliferation |
As wound inflammation subsides, collagen deposition can begin, resulting in increased wound tensile strength. Wound strength early in the inflammatory phase is minimal and based on fibrin clot and early epithelialization. At the end of the inflammatory phase, approximately 5 to 7 days after injury, a wound has only approximately 10% of its final tensile strength.
Proliferative Phase
The proliferative phase is the next major step in wound healing, and it is characterized by:
- •
Re-epithelialization
- •
Neovascularization
- •
Collagen deposition
- •
Wound contraction.
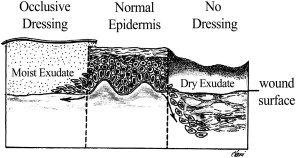
Re-epithelialization begins within 24 hours of injury as epithelial cells from wound margins or deeper adnexal structures, such as hair follicles or sebaceous glands, migrate into the wound to re-establish a protective barrier over underlying tissues. Granulation tissue develops approximately 3 to 4 days after an injury, and it is composed of inflammatory cells, new blood vessels, and fibroblasts in a matrix containing fibrin, glycoproteins, collagen, and glycosaminoglycans. Granulation tissue serves as a scaffold for cell migration, and it is present until re-epithelialization is complete. Re-epithelialization occurs rapidly in wounds closed primarily but can take 3 to 5 days in a wound healing by secondary intention. A moist environment aids the movement of epithelial cells, with most rapid wound coverage occurring in occluded moist wounds. In contrast, migrating epithelium takes longer over desiccated or partially necrotic surfaces, leading to significantly impaired wound healing ( Fig. 2 ).
Fibroblasts are a critical cellular element in a healing wound’s proliferative phase. These cells generally appear approximately 2 to 3 days after an injury, and they replicate and migrate in response to mediators, including C5a, fibronectin, PDGF, FGF, and TGF-β. Fibroblasts are involved in the production of collagen, elastin, fibronectin, glycosaminoglycans, and collagenase, which is important in the later maturation and remodeling phase of wound healing. Collagen synthesis increases dramatically by the fourth day after an injury. Fibroblasts secrete procollagen that is cleaved to tropocollagen, which then aggregates into fibrils that combine to form the collagen fiber, where subsequent cross-linking aids in increasing local tissue strength. Type III collagen predominates in early wound healing, but type I collagen is the major component in mature scar tissue.
Wound contraction, also an integral component of the proliferative phase of wound healing, is mediated by myofibroblasts, which are differentiated fibroblasts. Maximal contraction occurs at 12 to 15 days at an average of 0.6 to 0.75 mm of movement a day. Contraction of a wound becomes more severe if there is significant inflammation present or if the wound is left open for an extended period. Wound contraction is lessened in the presence of skin grafts, but it is still significant, with full-thickness skin grafts commonly experiencing 20% contraction.
Remodeling/Maturation Phase
The final stage of wound healing is the remodeling or maturation phase. Type III collagen is gradually replaced by type I collagen, and the maturing scar becomes stronger while also decreasing in size and erythema. Further organization of collagen fibers into a more parallel fashion allows for increased tensile strength and an improved appearance. The neovascularization of the wound eventually regresses, leading to a relatively avascular mature scar. Final remodeling of a wound may take 12 to 18 months, and the scar will have, at maximum, 70% to 80% of the original tensile strength of healthy, unwounded skin.
Clinical considerations/diseases that impede healing
The physiology of optimal wound healing can be adversely affected by both local and systemic factors ( Table 2 ). Local factors having an impact on healing include
- •
Wound closure techniques
- •
Wound dessication
- •
Tissue ischemia
- •
Local infection.
| Systemic Factors | Local Factors |
|---|---|
Hereditary
Chronic pulmonary disease Liver failure Malignancy | Ischemia Infection Tissue trauma Retained foreign body desiccation Medications Glucocorticoids Anticoagulants Antineoplastic agents Colchicine Penicillamine Vitamin E Salicylates (high dose) Nonsteroidals (high dose) Zinc sulfate (high dose) Vitamin A (high dose) |
Many chronic medical conditions can result in suboptimal or impaired wound healing because of poor nutrition or immunologic function :
- •
Vascular conditions
- •
Metabolic disorders
- •
Immune deficiency states
- •
Chronic liver disease
- •
Diabetes mellitus
- •
Malignancies
- •
Thrombocytopenic conditions.
Although more rare, systemic genetic disorders, such as Ehlers-Danlos syndrome, cutis laxa, osteogenesis imperfecta, and progeria, can alter normal healing mechanisms. In addition, congenital errors in metabolism that result in defective collagen production have an impact on the later phases of wound healing.
Advanced age results in slower healing and decreased wound tensile strength. History of radiation therapy is also a known risk factor for poor wound healing. Systemic medications can have a significant clinical impact on the healing process. Corticosteroids inhibit aspects of the inflammatory phase and diminish collagen synthesis and wound contraction. Corticosteroids also decrease cellular defense mechanisms, resulting in increased infection risk. Similarly, certain chemotherapeutic medications can affect wound healing by altering the inflammatory response, collagen synthesis, or wound contraction.
Clinical considerations/diseases that impede healing
The physiology of optimal wound healing can be adversely affected by both local and systemic factors ( Table 2 ). Local factors having an impact on healing include
- •
Wound closure techniques
- •
Wound dessication
- •
Tissue ischemia
- •
Local infection.
| Systemic Factors | Local Factors |
|---|---|
Hereditary
Chronic pulmonary disease Liver failure Malignancy | Ischemia Infection Tissue trauma Retained foreign body desiccation Medications Glucocorticoids Anticoagulants Antineoplastic agents Colchicine Penicillamine Vitamin E Salicylates (high dose) Nonsteroidals (high dose) Zinc sulfate (high dose) Vitamin A (high dose) |
Many chronic medical conditions can result in suboptimal or impaired wound healing because of poor nutrition or immunologic function :
- •
Vascular conditions
- •
Metabolic disorders
- •
Immune deficiency states
- •
Chronic liver disease
- •
Diabetes mellitus
- •
Malignancies
- •
Thrombocytopenic conditions.
Although more rare, systemic genetic disorders, such as Ehlers-Danlos syndrome, cutis laxa, osteogenesis imperfecta, and progeria, can alter normal healing mechanisms. In addition, congenital errors in metabolism that result in defective collagen production have an impact on the later phases of wound healing.
Advanced age results in slower healing and decreased wound tensile strength. History of radiation therapy is also a known risk factor for poor wound healing. Systemic medications can have a significant clinical impact on the healing process. Corticosteroids inhibit aspects of the inflammatory phase and diminish collagen synthesis and wound contraction. Corticosteroids also decrease cellular defense mechanisms, resulting in increased infection risk. Similarly, certain chemotherapeutic medications can affect wound healing by altering the inflammatory response, collagen synthesis, or wound contraction.
Creating the optimal environment for healing
Nutrition
The process of healing places the body into an anabolic state that requires additional energy intake; therefore, the first step to improving healing conditions should begin with assessing the health of the patient. Serum albumin is an essential tool in assessing nutritional status. The Veterans Administration cooperative study of preoperative risk factors and adverse outcomes linked low serum albumin levels to increased risk for wound dehiscence or wound complications. These findings emphasize the need for adequate essential amino acid supply and protein synthesis. Deficiency in protein synthesis has been recognized as delaying healing through suppression of fibroblast proliferation, angiogenesis, collagen synthesis, and collagen remodeling.
Although providing supplemental nutrition for diseased or malnourished patients to improve healing is commonplace, few studies have examined the effects of nutritional supplementation on wound healing in healthy individuals. A randomized controlled crossover trial that provided an oral vitamin supplement (containing proteases, bromelain, vitamin C, calcium, rutin [bioflavanoids], and grapeseed extract) revealed accelerated healing in the supplemented patient group when compared with the control group. Although the study suggested more rapid healing, no comment was made on the final aesthetic outcome of the scar. These data support the concept that several vitamins play essential roles in proper healing. Vitamin A is important for the cross-linking and synthesis of collagen, with deficiencies causing inhibition of epithelialization and delayed wound closure. Likewise, vitamin C is required for the proper hydroxylation of proline and lysine during collagen cross-linking. Without this hydroxylation step, the collagen molecule would be unstable, providing no tensile strength and little resistance to enzymatic breakdown. Vitamin C also serves an important role in neutrophil function as a reducing agent in superoxide radical formation. The vitamin B family also participates in collagen cross-linking. Zinc is involved in the enzymatic activity of RNA and DNA synthesis and collagenase function. Deficiencies in zinc cause delays in epithelialization and fibroblast formation. In contrast, vitamin E has been shown to reduce collagen production and tensile strength. For this reason, vitamin E has become a popular topical cream to prevent scar hypertrophy, although there is lacking statistical evidence that application of vitamin E actually improves scars. Although there are no definitive studies proving that supplementation of vitamins enhances healing in healthy individuals, there are data that vitamin deficiencies have a negative impact on the healing process. For this reason, the nutritional status of a patient should always be assessed, especially in the setting of a poorly healing wound.
Cleaning Agents/Cleaning Wounds
When selecting a wound cleaner, its cleaning capacity must be weighed against the potential toxicity of the cleaning agent to the cells within the wound bed. The utility of antiseptics on intact skin is well established and broadly accepted; however, the application of antiseptics to open wounds, especially those containing detergents, has been controversial secondary to potential cytotoxic effects on fibroblasts, keratinocytes, and leukocytes within the wound.
Hydrogen peroxide is a popular antiseptic cleansing agent, but its use in open wounds continues to be controversial. A review of the literature evaluating both in vitro and in vivo studies revealed that although 3% hydrogen peroxide had low toxicity on tissues, it showed poor ability to reduce the bacterial load within wounds. This review concluded that hydrogen peroxide is safe for use in open wounds and may provide some mechanical benefit in loosening debris and necrotic tissue, but it may be inefficient as an antiseptic.
One of the most common agents used in wound cleaning is 0.9% sodium chloride (saline). It is widely accepted as a gentle means for cleansing all types of wounds. Additionally, a Cochrane collaboration study suggested water, although not isotonic, is an acceptable alternative method to saline. (Cochrane studies are systematic reviews performed by health care providers that analyze primary research publications to objectively provide evidenced-based decisions on a particular topic. The Cochrane Collaboration, a nonprofit organization that is independent from financial interests, upholds a nonbiased opinion on an evaluated topic.)
In the setting of contaminated wounds, multiple methods for washing out debris have been described, including high-pressure pulsed lavage irrigations and bulb syringe irrigations. When compared directly, high-pressure pulsed lavage irrigation demonstrated superior results in the removal of contaminants. Irrigation pressures of 10 psi and 15 psi are more effective at removing bacteria and debris than 1 psi and 5 psi. Irrigation pressures of 20 psi or greater did not show increased efficacy and raised concern about potentially causing penetration of debris deeper into the wound. Regardless of the method of irrigation delivery, copious amounts of irrigant should be used to remove contaminants and bacteria. Irrigants commonly used include saline or antibiotic-infused saline (such as 50,000 units of bacitracin to 1 L of saline).
Surgical Techniques for Optimizing Wound Healing
To optimize wound healing, several guidelines exist to direct the proper employment of surgical technique ( Box 2 ). These include the tenets set forth by renown surgeon Dr William Stewart Halsted :
- 1.
Gentle handling of tissue
- 2.
Aseptic technique
- 3.
Sharp anatomic dissection of tissue
- 4.
Careful hemostasis
- 5.
Obliteration of dead space
- 6.
Avoidance of tension
- 7.
Reliance on rest.