Pancreas procurement for transplantation consists of removal of the entire pancreas along with the duodenum and spleen for whole organ transplantation.
Pancreas procurement for islet isolation consists of the removal of the pancreas from a deceased donor with the objective of isolating pancreatic islets for transplantation into type 1 diabetic recipients that meet the criteria for the procedure (Table 1). Pancreatic islet isolation involves the enzymatic and mechanical digestion of the pancreas followed by the purification of islets from the surrounding exocrine tissue. Isolated islets are then cultured and transplanted into type 1 diabetic recipients by either guided percutaneous infusion or direct infusion via the portal vein.1
A thorough history of the donor must be obtained and reviewed by qualified personnel on the transplant team.
Donor history of cancer and infectious disease and donor social history must be assessed for risk of transmission to the recipient.
Variables that have also been associated with whole pancreas graft function are pancreas obtained from donors after brain death (DBD), normal renal and hepatic function tests, amylase and lipase levels within normal range, and low vasopressor requirements.
Several variables have been shown to help determine pancreas suitability for islet isolation. Among the consistent predictors of islet isolation success are nondiabetic patients with an HbA1c level less than 6%, donor age (<45 years), high body mass index (BMI) (>30), cold ischemia time (<10 hours), an intact organ after organ procurement with preservation of the pancreatic capsule,2 and use of University of Wisconsin (UW) preservation solution for organ preservation.3
Table 1: Indications for Pancreatic Islet Transplantation
Subjects should be men or women >18 years of age who have had documented type 1 diabetes mellitus for at least 5 years prior to enrollment in the study. There should be documentation of absent or very low C-peptide (e.g., absent basal C-peptide or stimulated C-peptide <0.3 ng/mL).
The distributions of body weight and body mass index (BMI) should be representative of the intended treatment population, subjects with brittle type 1 diabetes. Similarly, the baseline daily insulin requirements should generally conform to those of the target population. It is best to exclude patients with extremes of body weight or insulin requirements.
Subjects should have a documented history of severe hypoglycemia, metabolic instability, or both. The following are metabolic parameters that may be used for documentation of metabolic instability and hypoglycemia. You need not use every one of these parameters or should you be restricted to this list alone. We suggest that you discuss the following specific details with us during an end-of-phase 2 meeting:
The number of severe hypoglycemic events (e.g., hypoglycemia requiring assistance of another individual) during the year prior to enrollment
Quantification of hypoglycemia and metabolic lability using, for example, the hypoglycemic score (HYPO score) and Lability Index
Measurement of hypoglycemia unawareness using the Clarke scoring system
24-hour studies of the mean amplitude of glucose excursion
History of frequent hospital admissions for diabetic ketoacidosis
Pancreata obtained from donors after cardiac death (DCD), young age (<18 years of age), cardiac arrest prior to donation, sustained hyperglycemia, and prolonged cold storage have been shown to negatively correlate with isolation and whole graft and islet transplant outcome.
Donor and recipient must be ABO compatible and human leukocyte antigen (HLA) typing and crossmatching for T and B cells must be performed.4
A full laboratory assessment of the donor must be performed. Important tests include electrolytes, renal and liver function tests, amylase, lipase, glucose level, complete blood count, HbA1c, and serologies for cytomegalovirus (CMV), hepatitis B and C, HIV, syphilis, and Epstein-Barr virus (EBV).
Use a third-generation cephalosporin as preoperative prophylaxis to prevent the risk of posttransplant infection.
Decontaminate the gut by administration of amphotericin B, tobramycin, and ceftriaxone via nasogastric tube placement prior to organ recovery.
Usually, the pancreas and the liver are recovered “en bloc” and the organs are separated on the back table following retrieval. Techniques used for this procedure minimize potential injuries that could render the pancreas or the liver unsuitable for transplantation.
A median laparotomy is the preferred approach for recovery of abdominal organs. If multiple organs are being recovered, a combined median laparotomy and thoracotomy is indicated.
The peritoneum is incised and dissected starting at the mesentery of the terminal ileum, and the dissection continues through the peritoneal duplicature lateral to the right colon from the cecum proximally to the distal duodenum.
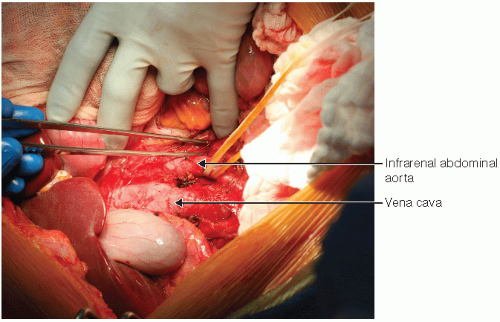
The right and transverse colon are dissected free from the retroperitoneum and moved to the left upper abdominal quadrant. The abdominal aorta, iliac arteries, inferior mesenteric artery, and vena cava are dissected and exposed.
The distal vena cava and distal aorta are looped with wet umbilical tapes and the aorta is circumferentially mobilized in preparation for cannulation and perfusion with cold preservation solution (FIG 1).
The gastrocolic ligament is transected, releasing the transverse colon from the greater gastric curvature and allowing exposure of the pancreas (FIG 2).
The splenic flexure of the colon is then mobilized and the short gastric vessels ligated and divided (FIG 3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree