Procurement of Pediatric En Bloc Kidneys
Christoph Troppmann
DEFINITION
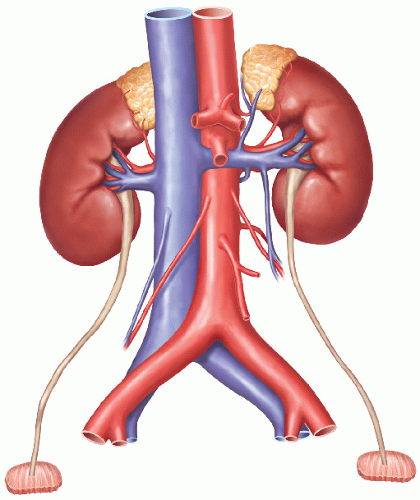
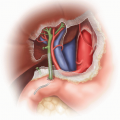
This chapter describes the recovery of pediatric donor kidneys as a single anatomic unit (i.e., leaving them attached to the donor’s abdominal aorta and inferior vena cava [IVC]) in preparation for transplanting these kidneys as a single unit (“en bloc”) into one recipient. The weight of pediatric en bloc kidney donors ranges typically from 2.5 kg to 25 kg. The goal of this surgical approach is to provide adult recipients with sufficient graft nephron mass while minimizing the technical challenges and complication rates that would result from transplanting these small kidneys as single grafts.
The decision to allocate kidneys from small pediatric donors as single versus en bloc kidneys is made by the hosting local organ procurement organization (OPO).
The majority of kidneys from donors between 10 and 20 kg of weight are transplanted en bloc. In selected cases, however, kidneys recovered en bloc can be split for transplantation as single kidney into two recipients (for instance, when kidney length exceeds 6 cm). In doing so, one must carefully weigh the principles of maximizing kidney graft availability (i.e., splitting the kidneys and providing a graft for two recipients) versus minimizing technical-vascular complications (i.e., leaving kidneys en bloc).1,2,3,4,5,6
En bloc graft back-table preparation and hypothermic pulsatile perfusion as well as donor and recipient selection criteria are described in Chapter 10.
SURGICAL MANAGEMENT
The principles and recovery techniques outlined in this chapter apply to both brain-dead and donation after cardiac death (DCD) donor recoveries, unless specifically noted otherwise.
En bloc kidney grafts have limited functional reserve until they have grown to meet the recipients’ needs. This limited reserve may be further compounded by other nonmodifiable factors (e.g., preterminal acute kidney injury, DCD donor status, extended warm and cold ischemia time). Hence, organ preservation must be optimized.
Use University of Wisconsin (UW) preservation solution (SPS-1®; Organ Recovery Systems, Inc, Itasca, IL) for recovery of en bloc kidneys from both brain-dead and DCD pediatric donors whenever possible.
Particularly for small and very small (<5 kg) en bloc donors, prerecovery communication and planning with all potentially involved extrarenal recovery teams are paramount. At that time, address the following important topics: flush and preservation solution to be used, the level where suprarenal aorta and IVC are to be divided, the venous venting site, and the insertion site of the abdominal aortic flush cannula.
Ensure prior to the start of the recovery operation that aortic flush cannulas of appropriate sizes are available and that proper brain death and DCD-related documentation and consent were obtained.
TECHNIQUES
BRAIN-DEAD DONORS—SURGICAL APPROACH FROM INCISION TO AORTIC FLUSH
Recovery of kidneys from pediatric brain-dead donors proceeds in principle as outlined in Chapter 1 for adult brain-dead donors.
Perform abdominal and thoracic (sternal) incisions as described for adult deceased donors. Extensively mobilize the right colon and small intestine (Cattell-Braasch maneuver).
Avoid dissecting into, and entering, retroperitoneal tissues anterior, lateral and inferior to the aorta and IVC to minimize the risk for injury to renal arteries and veins, hilar structures, and ureters.
With minimal dissection, identify only the superior aspect of the right and left renal vein confluence with the IVC.
Do not dissect the adrenal and gonadal veins.
Dissect the superior mesenteric artery (SMA) close to its aortic takeoff and place a vessel loop around it. The SMA is an important landmark for later aortic transection during combined kidney-liver and kidney-intestine(-liver) recoveries.
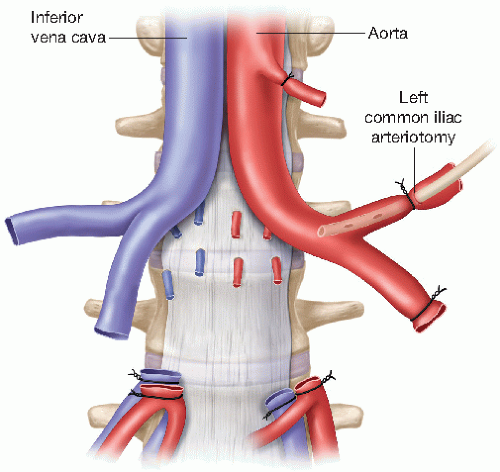
Determine the location of the aortic bifurcation and both common iliac arteries by inspection and palpation. Dissect and expose both common iliac arteries. One of the two common iliac arteries requires only limited exposure in order to facilitate its ligation at a later stage. Expose a longer segment of the contralateral common iliac artery in preparation for the eventual insertion of the aortic flush cannula (FIG 1). Expose enough length of that common iliac artery so that you can tie it off distally and can place a second tie around it, proximal to the future arteriotomy flush cannula insertion site (FIG 2).
Dissect the supraceliac abdominal aorta in preparation for aortic cross-clamping.
Mobilize the left colon and splenic flexure to allow for effective topical cooling of the left kidney during and after the aortic flush. Expose only the anterior surface of the left kidney and do not dissect and enter the planes posterior to the kidney at this stage of the recovery operation (to minimize the potential for traction injury on the renal vascular pedicle). Similarly, do not mobilize the right kidney posteriorly.
When all recovery teams are ready to proceed to aortic cross-clamp and flush, administer an intravenous (IV) bolus of heparin (500 to 1,000 U/kg).
Tie off the common iliac artery that is not used for the cannula insertion.
Tie off the distal contralateral common iliac artery. Create an arteriotomy proximal to the tie and insert the arterial cannula for the aortic flush (FIG 2). If a small flush cannula is not available, and depending on the donor’s common iliac artery diameter, use the beveled cut end of an IV tubing for the arterial cannulation.
Start the flush, preferably with UW preservation solution, using a total volume of 1 to 3 L depending on donor weight.
During the flush, venting of the central venous system into the chest is preferred. If a thoracic recovery team declines that option, incise one of the distal external iliac veins after aortic cross-clamp to ensure proper venting.
Ensure that the kidneys are properly topically cooled with ice slush during flush and organ excision.
SURGICAL APPROACH FROM AORTIC FLUSH TO EN BLOC KIDNEY REMOVAL— KIDNEY-ONLY RECOVERY
Create a large window in the left colonic and sigmoid mesentery by transecting the mesentery in the vicinity of the intestine, avoiding intestinal injury. Reflect the mesentery medially. This anterior approach significantly facilitates mobilization and dissection of the left kidney: There is no need to mobilize and move the entire left-sided colon and mesentery medially to gain access to the left kidney from a posterolateral direction. The left colon can remain in the left colic gutter or can easily be reflected away from the left kidney in a medial direction.
Dissect both ureters, including a wide swath of periureteral tissue, from their respective common iliac artery crossover point toward the bladder, using a no-touch technique for the ureters. In order to minimize ureteral trauma, do not encircle the ureters with a finger, umbilical tape, or vessel loop. Gently manipulate ureters and periureteral tissues as much as possible with forceps only. Do not futher dissect ureters and periureteral tissues craniad to their common iliac artery crossover.
After dissection and mobilization of the distal ureters, incise the bladder and excise each ureter from the bladder, including a small patch of bladder with each ureter if possible. Some authors have proposed the recovery (and transplantation) of small pediatric en bloc donors’ ureters on a common patch of donor bladder.10,11,12 This technique, however, awaits exact delineation of its potential benefits in future studies.
Mobilize the kidneys, remaining away from the central blood vessels (aorta, IVC) and the ureters. Dissect the lateral and posterior aspect of each kidney without placing traction on the kidneys or using them as a handle. Use a forceps to gently handle and lift each kidney as the lateral and posterior dissection proceeds in a caudocranial direction, encompassing the adrenal glands which must be left attached to the kidneys.
Transect the noncannulated, ligated common iliac artery distal to the tie as well as the contralateral common iliac artery between the distal tie and the flush cannula insertion site (FIG 2). Transect both common iliac veins at least 5 mm distal to the IVC bifurcation to facilitate creation of an IVC bifurcation branch patch for the venous anastomosis during the recipient operation.
Gently lift both proximal common iliac arteries and veins with a forceps and start the central (midline) dissection posterior to the aorta’s and IVC’s bifurcation, exposing the anterior longitudinal ligament of the spine. Use a tenotomy or Metzenbaum scissors, with the curve of the scissors tips pointing posteriorly (i.e., toward the operating table) to prevent injury to retroaortic and retrocaval vascular structures (i.e., the right renal artery and any retro- or circumaortic left renal veins) that may be encountered during this phase of the dissection (FIG 2). Reflect both ureters with their periureteral tissues in a craniad direction during this phase of the dissection to avoid inadvertent (peri-)ureteral injury.
Recover as much of the suprarenal aorta and IVC in continuity with the graft as possible, including (in the absence of a thoracic organ recovery) the thoracic aorta and retrohepatic IVC.
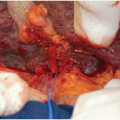
Remove the kidneys en bloc from the donor’s body and place them on ice slush. Do not perform any further dissection of the en bloc graft in the donor operating room. Leave the bifurcations of aorta and IVC as well as any iliac vessels attached in continuity with the graft. One of the common iliac arteries can be used for insertion of the machine perfusion cannula without having to sacrifice any distal aorta for that purpose.13 The bifurcations are important for fashioning branch patches during the transplant operation. Inspect and measure the kidneys. Measurement of kidney dimensions and inspection of flush quality are usually not hampered by the presence of Gerota’s fascia and the sparsely developed perinephric fat in these small donors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree