16 Pressure sores
Synopsis
 Pressure sores are a common problem associated with great morbidity and cost.
Pressure sores are a common problem associated with great morbidity and cost.
 They have been recognized since antiquity, though effective surgical treatment did not evolve until the 19th century.
They have been recognized since antiquity, though effective surgical treatment did not evolve until the 19th century.
 The pathogenesis involves not only pressure, but a multitude of additional factors including friction, shear, moisture, nutrition, and infection.
The pathogenesis involves not only pressure, but a multitude of additional factors including friction, shear, moisture, nutrition, and infection.
 A full evaluation of these factors, in addition to characterizing the wound in terms of stage, size, and involvement of the underlying bone, directs further treatment.
A full evaluation of these factors, in addition to characterizing the wound in terms of stage, size, and involvement of the underlying bone, directs further treatment.
 Prevention and treatment of pressure sores should focus on correcting these risk factors, reserving operative intervention until the patient is optimized.
Prevention and treatment of pressure sores should focus on correcting these risk factors, reserving operative intervention until the patient is optimized.
 Less severe pressure sores can often be treated with wound care and patient optimization.
Less severe pressure sores can often be treated with wound care and patient optimization.
 Thorough debridement, particularly of involved hard tissues, is critical, as with any wound.
Thorough debridement, particularly of involved hard tissues, is critical, as with any wound.
 While flaps based on the gluteus muscles, the tensor fasciae latae, and the hamstring are workhorse flaps, a vast array of surgical options exist to treat any given wound, assuming some basic principles of flap design are respected.
While flaps based on the gluteus muscles, the tensor fasciae latae, and the hamstring are workhorse flaps, a vast array of surgical options exist to treat any given wound, assuming some basic principles of flap design are respected.
 Recurrence rates are high even in the best of hands, and should be planned for preoperatively.
Recurrence rates are high even in the best of hands, and should be planned for preoperatively.
Introduction
Epidemiology
In 1999 Amlung et al.1 performed a 1-day pressure ulcer prevalence survey of 356 acute care facilities and 42 817 patients. The overall pressure ulcer prevalence rate was 14.8%; facility-acquired ulcers accounted for 7.1%. Ten years later, VanGilder et al.2 reviewed the results of the International Pressure Ulcer Prevalence Survey (IPUPS) and found overall prevalence and facility-acquired ulcer rates of 12.3% and 5% respectively. Overall prevalence rates were highest in long-term acute care facilities (22%), while facility-acquired rates were highest in adult intensive care units (ICUs), ranging from 8.8% in general cardiac care units to 12.1% in medical ICUs. A total of 3.3% of ICU patients developed severe ulcers, while 10% were device-related. A broader inquiry,3 reviewing over 400 000 records from the survey between 1989 and 2005, found overall and nosocomial pressure ulcer prevalence rates of 9.2% in 1989 and 15.5% in 2004. Rates were higher in long-term acute care facilities, at 27.3%. Overall, pressure ulcer prevalence appears relatively stable despite significant advances in treatment and prevention.
The prevalence of pressure ulcers in the nursing home population has been reported to be anywhere from 2% to 28%.4,5 Data from the 2004 National Nursing Home Survey revealed an overall 11% prevalence rate, with elderly residents, residents with recent weight loss, and short-term residents being at even higher risk.6 The reported prevalence of pressure ulcers in long-term acute care facilities is likewise variable, ranging from 5% to 27%,7,8 while the IPUPS reported an overall 22% rate in this setting.
Particular populations have been identified at particularly high risk. There is a strong association between hip fractures and pressure sores, though incidences have been reported from 8.8% to 55%. A large European study9 revealed an overall prevalence of 10% on arrival and 22% at discharge in this population, with dehydration, advanced age, moist skin, higher Braden scores, diabetes, and pulmonary disease all associated with higher rates of ulceration. The majority were stage I and none was stage IV. Baumgarten et al.10 reported an 8.8% incidence of hospital-acquired pressure sores in a study involving multiple centers in the US. Another study by the same group,11 with rigorous surveillance for ulcers in a slightly older population, revealed an incidence of 36.1%. Incidence was highest in the acute care setting and associated with longer wait before surgery, ICU stay, longer surgery, and general anesthetic.
Spinal cord injury (SCI) patients are at particular risk for pressure sores due to the combination of immobility and insensitivity. Rates have been reported to be 33–60%12–14 and it is the second leading cause of rehospitalization after SCI. Gelis et al.15 reviewed pressure ulceration in the SCI population and noted a 21–37% prevalence in the acute stage, a 2% rate leaving a rehabilitation center, and a 15–30% rate in the chronic stage. Identified risk factors included race, history of previous ulceration, and tobacco use.
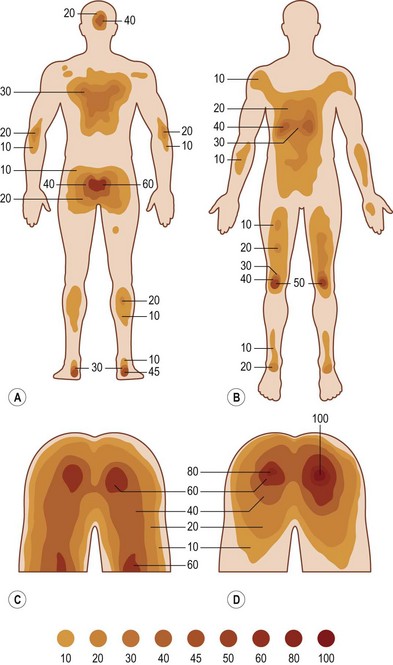
Anatomic distribution
In 1964 Dansereau and Conway16 published the results of their evaluation of 649 patients from the Bronx Veterans Administration Hospital with 1604 pressure sores among them. The authors noted that the vast majority of pressure sores developed in the lower part of the body, the ischial tuberosity being the most common site, accounting for 28% of all ulcers. In Meehan’s 199417 review of 3487 patients with 6047 pressure sores, the most common site of occurrence was the sacrum (36%), followed by the heel (30%). More recently VanGilder et al.2 reported that the sacrum (28.3%) and the heel (23.6%) were the most common sites for pressure ulceration, followed by the buttocks (17.2%).
Costs
The National Pressure Ulcer Advisory Panel (NPUAP) has estimated the cost to treat and heal hospital-acquired pressure sores to be up to $100 000 per patient.18 If the additional costs, both surgical and nonsurgical, of managing pressure sores at nursing homes and home care facilities are considered, the financial burden is estimated by the Institute for Healthcare Improvement at approximately 11 billion dollars in 2006.
A review from 2006 of the Healthcare Cost and Utilization Project19 revealed 503 300 hospital stays during which pressure sores were noted. Patients with pressure sores were inpatients nearly three times longer than patients without such a diagnosis (14.1 days versus 5.0 days). Patients with pressure sore as a primary diagnosis cost an average of $1200 a day, while patients with pressure sore as a secondary diagnosis cost an average of $1600 a day, compared with an average of $2000 a day for all other conditions. Mean cost per hospitalization was $16 800 and $20 400 for stays with a principal and secondary diagnosis of pressure sore, versus only $9900 for all other conditions. Aggregate costs were calculated to be $752 million and $10.2 billion for stays with primary and secondary diagnosis of pressure sore respectively.
Historical perspective
Pressure sores are an ancient problem, observed at autopsy of Egyptian mummies.20 As early as the 16th century, Ambrose Paré21 had recognized the importance of pressure and nutrition in the treatment of these lesions. By 1873 Sir James Paget correctly surmised that pressure led to compression of the cutaneous vasculature, leading to necrosis.22 Though he incorrectly attributed them to central nervous system lesions, in 1878 Charcot23 provided a detailed description of what he termed decubitus ominosus, noting not only the clinical manifestations of the disease but the poor prognosis they portended. Though well over a century old, Charcot’s description could be applied to any number of modern patients with pressure sores of various stages:
John Staige Davis24 was the first to suggest replacing the unstable scar of a healed pressure sore with flap tissue in 1938. Lamon25 described the first closure of open pressure sores in 1945, and by the end of the decade most of the surgical manipulations of pressure sores as we know them today had been reported.
Kostrubala and Greeley26 recommended excision of the bony prominence and padding the exposed bone with local fascia or muscled fascia flaps in 1947. That same year, Conway et al.27 emphasized the importance of tissue laxity at the reconstructed site to allow for joint motion and recommended skin-grafting the donor sites of local rotation flaps used for coverage. In 1948 Bors and Comarr28 used the gluteus maximus as a muscle rotation flap, and the next year Blocksma et al.29 rotated the biceps femoris muscle stump to cover the exposed bone following total ischiectomy.
Basic science
Pressure
As mentioned previously, Paget described the connection between pressure and decubitus ulcers as early as the 19th century. Though many other factors may contribute to their formation, pressure sores are thought to result from pressure applied to soft tissue at a level higher than that found in the blood vessels supplying that area for an extended time period. In 1930 Landis30 performed a classic series of experiments and found that the average capillary closing pressure is approximately 32 mmHg. Fronek and Zweifach31 reported similar findings in their study, noting perfusion pressures between 20 and 30 mmHg (Fig. 16.1).
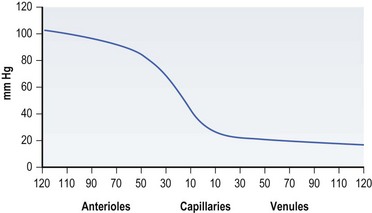
Fig. 16.1 Pressure in various components of the tissue microcirculation (diameter in µm).
(Data from Fronek K, Zweifach BW. Microvascular pressure distribution in skeletal muscle and the effects of vasodilation. Am J Physiol 1975;228:791; reprinted with permission from Woolsey RM, McGarry JD: The cause, prevention, and treatment of pressure sores. Neurol Clin 1991;9:797.)
Lindan et al.32 documented the human distribution of pressure points by means of a “bed of springs and nails.” With the subject supine, the points of highest pressure were the sacrum, buttocks, heel, and occiput, all of which were subject to pressures of roughly 50–60 mmHg. When sitting, pressures up to 100 mmHg were recorded over the ischial tuberosities. Subjects in Lindan’s study were tested on surfaces that were significantly softer than comparable wheelchair seats of beds, but even on padded surfaces with wide load distributions, all major weight-bearing areas sustained pressures in excess of end-capillary pressures (Fig. 16.2).
However, simply applying pressure in excess of these levels does not necessarily result in tissue ischemia. Much of the pressure applied to tissues is carried by the connective tissues surrounding the blood vessels. Furthermore, autoregulation of local blood flow will tend to increase blood pressure in response to applied pressure within a certain range.30,33
Efforts have been made to quantify the degree of pressure necessary to cause tissue damage. Dinsdale34 found that pressure roughly double capillary closing pressure, applied for 2 hours, resulted in irreversible ischemic damage to tissue. Pressures below this threshold were unlikely to cause tissue necrosis, while increased pressures were correlated with increased likelihood of ulceration. Kosiak et al.35 noted similar findings in dog tissues, but noted that if the pressure was released every 5 minutes, few changes occurred. Groth36 published a detailed study on the relationship between applied pressure and the onset of tissue damage in a rabbit model, noting an inverse relationship wherein higher pressures caused damage in less time. Husain37 had similar results in a rat model, also noting that pressure applied over a large area was less injurious than when applied over a smaller one.
Furthermore, various tissues have different susceptibility to pressure. Nola and Vistnes38 noted that pressure on skin over a bone is more injurious than pressure on skin over muscle. On the other hand, Daniel et al.39 noted that muscle was more susceptible to injury than skin, requiring less pressure for a shorter duration likely due to its increased metabolic activity.
Friction
Friction is the force resisting relative motion between two surfaces, and is the precursor to shear. It develops between the patient’s skin and any number of contact surfaces, including the patient’s bedding, transfer devices such as sheets, rollers, or slide boards, various appliances and orthotics, and mobility devices such as wheelchair cushions. Excess friction may result in superficial skin injury such as abrasions, blisters, and even skin tears in patients with fragile skin.40,41 While relatively minor in isolation, such injuries may potentiate further damage. As the integrity of the skin is compromised, transepidermal water loss increases and allows moisture to accumulate. Moisture in turn increases the coefficient of friction and promotes adherence to sheets and other contact surfaces.42
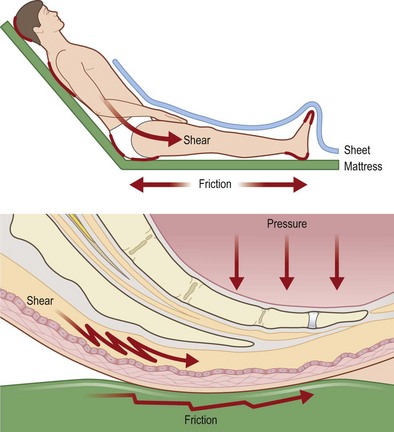
Shear (Fig. 16.3)
Reichel43 was one of the first authors to describe shear as a risk factor for developing decubitus ulcers, noting that patients developed more sacral pressure sores when the head of their bed was elevated. Shear develops when friction adheres skin and superficial tissues to sheets or bedding which are then stretched tightly over deeper structures. The underlying blood vessels are then stretched, angulated, and may be injured by this stress. Subcutaneous tissue in particular lacks tensile strength and is particularly susceptible to shear stress.44 Dinsdale34 noted that addition of shear forces greatly decreased the amount of pressure needed to cause ulceration in a pig model, concluding that “a shear force is more disastrous than a vertical force.” Goossens et al.45 noted similar results in human subjects, finding that the addition of a small shear component drastically reduces the level of pressure needed to cause critical ischemia over the sacrum.
Patient transfers, sliding or dragging the patient in bed, “boosting” patients up in bed, or allowing patients to elevate themselves in bed by pushing with elbows and heels all cause significant shear.41 Certain positions also cause elevated levels of shear: patients in the semi-Fowler’s position or sliding down in a wheelchair both experience significant shear over the lower back and buttocks.46 This can explain why wheelchair-bound patients may still develop a sacral pressure sore if they are not sitting upright in their chair, despite the fact that theoretically the ischial tuberosities should be bearing the majority of their weight.
Moisture
Excessive moisture is not only a risk factor for pressure sores, but may also result in a separate pathology inclusive of incontinence dermatitis, perineal dermatitis, and moisture lesions.47 Whether a separate entity resulting in skin breakdown due primarily to moisture and irritation as opposed to pressure is a legitimate or useful distinction is a matter of some debate.48,49 However, it is clear that moisture is an important factor to consider when evaluating pressure sores, and pressure relief should not be neglected even if lesions are thought to be primarily due to moisture. Moist skin has a higher coefficient of friction and is prone to maceration and excoriation.44
Though excess moisture can have many causes, urinary and fecal incontinence is of particular concern in the etiology of pressure sores. Incontinence is common in the elderly, and rates are even high in the institutionalized population, with rates from 20% to 77% for urinary incontinence50,51 and from 17% to 50% for fecal incontinence.52 In addition to causing excess moisture, urine also negates the acidic pH of skin through the introduction of nitrogen derivatives. Fecal contamination introduces a large bacterial load. Lowthian48 noted a fivefold increase in pressure sores in patients who were incontinent, though he did not distinguish between urinary and fecal incontinence. While some studies have found a relationship between urinary incontinence and pressure sores,53 others have failed to find a correlation while noting a significant correlation with fecal incontinence.54–56
While excess moisture is clearly deleterious, the opposite is also true. Excessively dry skin is prone to cracking, has decreased tensile strength and lipid content, and impaired barrier function, and appears to be an independent risk factor for pressure ulceration.57,58
Malnutrition
Patients who are chronically ill and debilitated frequently have accompanying nutritional deficiencies that manifest as low serum albumin, prealbumin, or transferrin levels. Prevalence rates range from 1% to 4% in elderly patients living at home, versus 20% in hospitalized patients and 37% in institutionalized patients.59 The deleterious effects of poor nutrition include weight loss, negative nitrogen balance, poor wound healing, and immunosuppression.60 Several studies have documented a clear association between protein malnutrition and wound healing,61–63 and patients who are severely malnourished are at increased risk of sepsis, infection, in-hospital mortality, and longer hospital stays.64,65 However, the relationship between malnutrition and pressure sore prevention, as opposed to treatment, is not entirely clear. There is certainly a strong correlation between malnutrition and pressure sores,66–68 but a clear causal link remains elusive.
Neurological injury
Despite extensive recommendations aimed at addressing the issue, pressure sores remain the most common complication69,70 and the second most common cause of hospital admission71 in the SCI population. Immobility, either in bed or in a wheelchair, leads to increased pressure, friction, and shear that is a causative factor in all pressure sores. However, SCI eliminates the protective sensation that would normally stimulate them to alter their position in response to prolonged pressure, particularly during sleep. Pressure which would be innocuous with intermittent relief leads to pressure sore development.46
In addition to the obvious problems of immobility and decreased sensation, incontinence, spasticity, and psychosocial issues are common problems in this population. Spasticity, a common but not inevitable sequela of SCI, is a problem unique to the SCI population, affecting 65–78% patients 1 year postinjury.72 Spasticity is characterized by hyperreflexia, clonus, and increased muscle tone. While it is not included in most pressure sore risk scales, it has effects due to direct increase in mechanical stress and altered weight distribution, as well as complicating patient positioning, skin inspection, and hygiene.73
Diagnosis
Classification
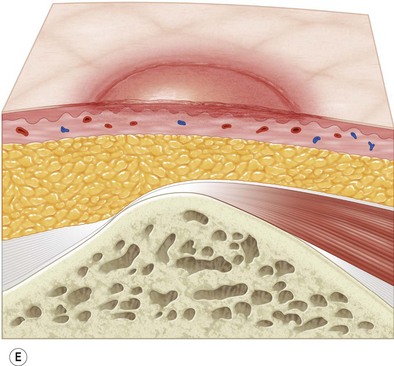
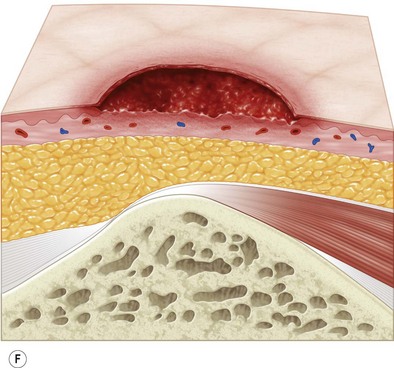
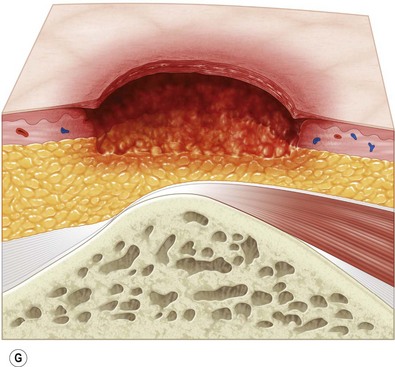
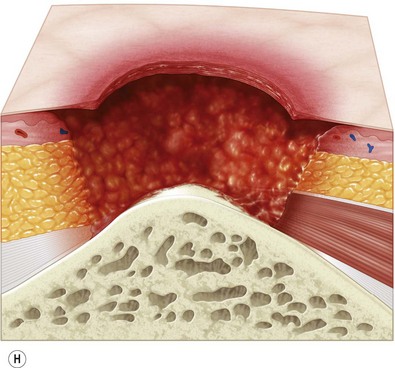
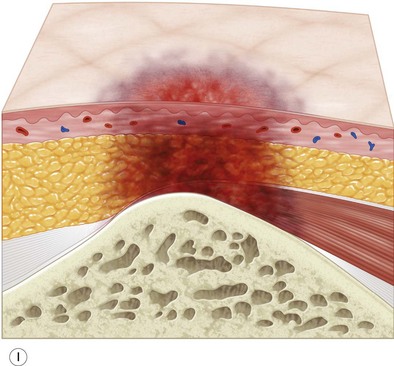
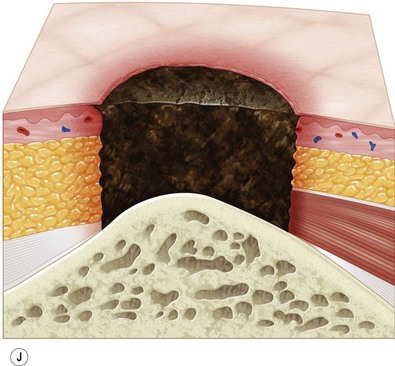
Multiple classification systems exist to describe pressure sores. The most commonly used system is the NPUAP staging system,74 a modification of Shea’s original classification,74,75 most recently revised in 2007 (Fig. 16.4). Though used in basic form for many years, two additional classifications, suspected deep-tissue injury and unstageable, have been added relatively recently to the NPUAP system.
Patient evaluation
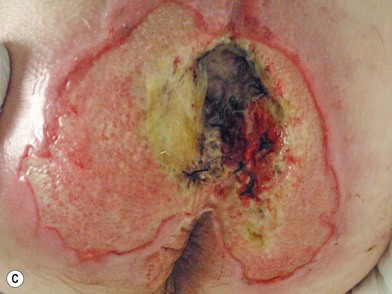
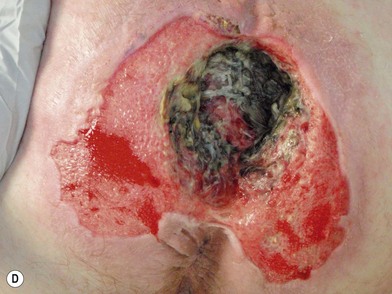
When evaluating a new patient with a pressure sore, a wide variety of factors must be considered. Both the wound and the patient should be meticulously examined. A wound history, noting the onset, duration, prior treatments and procedures, and wound care regimen, should be noted. Wounds should be measured in three dimensions, with notes made regarding any tunneling or undermining. The tissue at the margins of the wound should be examined for any signs of occult deep-tissue injury, infection, and scarring. The base of the wound should be characterized, noting the presence of eschar, slough, or other necrotic tissue. If necrotic material obscures the base of the wound, it should be debrided until a full assessment can be performed. Granulation should be noted in terms of both character and percentage of the wound bed covered. Exposed tissues, such as bone, tendon, or joint, should be noted. If easily accessible the bone can be characterized as hard, soft, or obviously necrotic. The amount and character of the wound exudate should be documented as well. A measuring tape and camera can improve documentation and make tracking progression easier.76
A reasonable initial set of studies would include a complete blood count, basic metabolic panel, creatinine, and blood urea nitrogen. Albumin and prealbumin should be obtained at the initial consult and regularly thereafter to evaluate for malnutrition and follow the progression of therapy. C-reactive protein and erythrocyte sedimentation rate (ESR) can be obtained to evaluate for osteomyelitis, though they will almost certainly be positive in deeper ulcers. The evaluation of the pressure sore patient for osteomyelitis is discussed in the next section. Wound cultures of the open pressure sore are of little value and should not be used to direct antibiotic therapy,77 as opposed to needle or surgical bone biopsy.
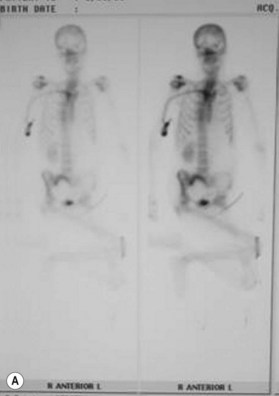
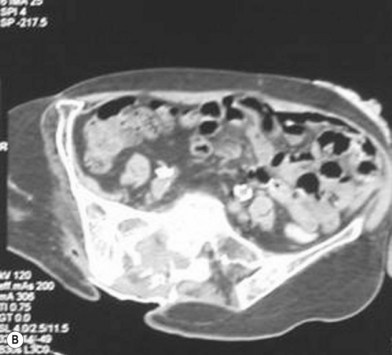
Osteomyelitis
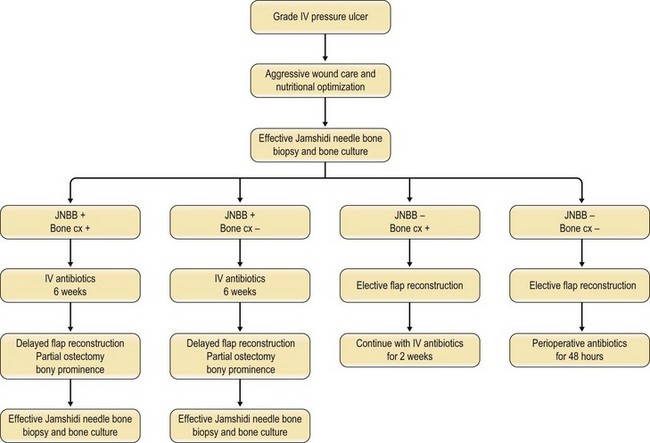
In 1970 Waldvogel et al.78 recognized that osteomyelitis is responsible for wound infection and breakdown after reconstruction of stage III and IV pressure sores (Fig. 16.5). Patients with pressure sores complicated by osteomyelitis have significantly longer hospitalizations than those who do not.79 Establishing the diagnosis of osteomyelitis is essential before embarking on definitive surgical treatment of pressure sores. Unrecognized osteomyelitis is a major source of morbidity and increased costs.79
In 1988 Lewis et al.80 tried to assess the value of some common test in diagnosing osteomyelitis by following 61 patients with pressure sores, 52 of whom eventually had confirmed histopathologic diagnosis of osteomyelitis from surgical specimens. This prospective trial examined white blood cell count, ESR, plain X-ray, technetium-99 m bone scans, computed tomography (CT) scan, and Jamshidi-needle bone biopsy. The authors felt that the most practical, revealing, and least invasive preoperative workup involved a combination of white blood cell count, ESR, and two-view X-ray. Only one positive test was needed to make the diagnosis. Sensitivity was 89% and specificity 88% using this protocol. Bone scans and CT scans were expensive and not very sensitive. Needle bone biopsy had a sensitivity of 73% and a specificity of 96%. Of note, magnetic resonance imaging (MRI) was not included in this analysis.
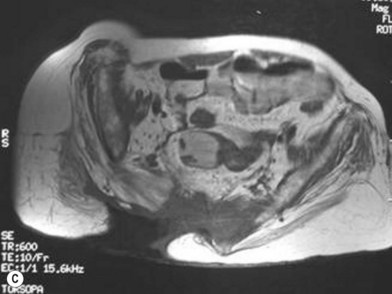
MRI has taken on a larger role in the diagnosis of osteomyelitis. Huang and colleagues81 analyzed 59 consecutive MRIs in 44 paralyzed patients and found an overall accuracy of 97%, sensitivity of 98%, and specificity of 89%. The authors concluded that not only was MRI accurate, it helps define the extent of infection, which may help limit the surgical resection. Ruan et al.82 independently confirmed these findings and feel that MRI is superior to CT for the evaluation of osteomyelitis.
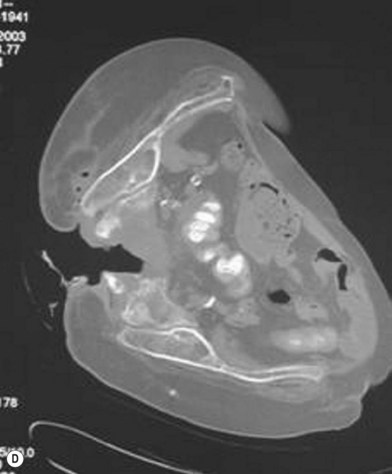
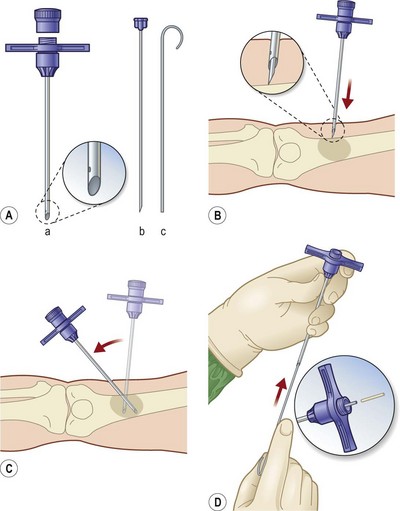
Han et al.79 reviewed their series of press ulcers and noted that their high osteomyelitis-related complication rate – largely consisting of deep abscess and sinus tracts – was a result of their inability to diagnose osteomyelitis accurately preoperatively. Their group moved to a two-stage procedure, performing wound debridement and Jamshidi core needle bone biopsy at the first operation (Fig. 16.6). If the biopsy results were positive, closure was delayed for 6 weeks while the osteomyelitis was treated with antibiotics; otherwise the wound was closed with a musculocutaneous flap. The authors also noted that bone cultures alone were not as useful as bone cultures and histopathology of the bone biopsy together, and found that the biopsy had a positive and negative predictive value of 93% and 100%, respectively.
These results are in keeping with several other studies that support the utility of bone biopsy in the diagnosis of osteomyelitis.83–86 Marriott and Rubayi87 have used the results of bone biopsy to determine the duration of antibiotic therapy, in some cases truncating treatment if pathology reveals only chronic osteomyelitis.
Psychological evaluation
Psychological problems are common in the pressure sore population. Langer88 finds 22% of patients with SCI had diagnosable depression, considerably higher than found in the general population. Frank et al.89 report 37.5% of patients with SCI had a diagnosis of major depression. Akbas et al.90 report 47.4% of patients with pressure sores fulfilled the diagnostic criteria for major depression. In their prospective study, they found the depression rates to be higher in women, younger patients, and those who had undergone multiple operations for their pressure sores. The authors recommend “intensive psychological counseling” in the treatment of the pressure sore patient. Foster et al.91 suggest that psychological factors play a significant role in predicting pressure sore recurrences.
Treatment
Prevention
Obviously, prevention of pressure sores is preferable to treatment. As healthcare moves towards nonpayment for nosocomial pressure sores, there is ever-greater incentive to avoid these lesions. However, despite extensive study and recommendations, overall pressure sores rates have changed little in recent years.3,92 Some pressure sores may not be preventable,93–95 and using pressure sores as an indicator of quality is a contentious issue, particularly in light of possible negligence litigation.96 However, though no strategy has been found which reduces pressure sore rates to zero, there are several measures, many of which now constitute standard care, which may contribute to pressure sore prevention.58
Risk assessment
The first step in pressure sore prevention is risk assessment. Multiple assessment scales have been devised, including those by Braden, Gosnell, Knoll, Norton, Waterlow, and Douglas.97 Several are designed for specific subpopulations and each has its merits. All suffer from potential inaccuracies as their use is dependent on the competence of its assessor and the setting in which it is used.
Norton98 developed an assessment scale for use in geriatric patients. The scale incorporates general physical condition, mental status, activity, mobility, and incontinence and ranges from 5 to 20, with lower scores associated with greater risk. Patients with a score of 11 or less had a 48% incidence of pressure sores, while those with a score of 18 or greater had a 5% incidence. Gosnell99 added nutritional status as a variable to his method, which is often referred to as the “modified Norton scale.”
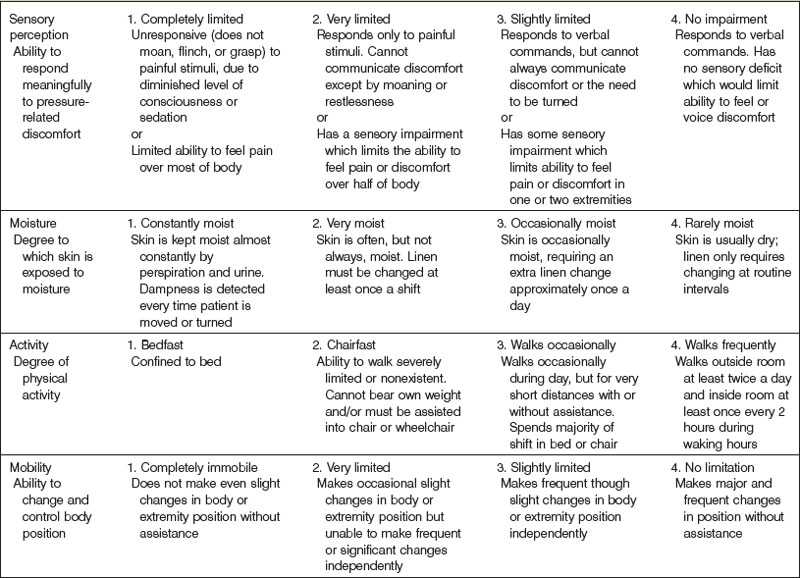
The most widely used pressure sore assessment tool is the Braden scale100 (Table 16.1). The Braden scale incorporates six subscales that assess sensory perception, skin moisture, activity, mobility, friction and shear, and nutrition. Scores range from 6 to 23, with higher scores associated with an increased risk of developing pressure sores. The original study noted a score of 16 to be the threshold for pressure sore development,100 though a different cutoff score may be more appropriate in different settings.101,102
Multiple studies have examined the validity, sensitivity, specificity, and predictive value of the various scales. Generally, assessment scales are superior to nurses’ clinical judgment when predicting future pressure ulceration.97 However, there is no evidence that use of risk assessment decreases pressure sore incidence.103–105 Whether this is because interventions are not instituted or are ineffective is unclear.104
Skin care (Box 16.1)
Ideal skin care encompasses cleaning, hydrating, protecting, and replenishing the skin as needed. Proper skin care can be time- and labor-intensive, and is at times neglected by physicians and nurses alike.106
Box 16.1
General skin care principles
Assess the patient’s skin daily
Cleanse skin when indicated using a pH-balanced cleanser
Minimize exposure to moisture (e.g., incontinence, wound leakage)
Use skin barrier product to protect vulnerable skin
Common recommendations for skin cleansing involve the use of warm soap and water followed by drying by rubbing or patting.107 Washing involves the use of soap and surfactants, which, while effective at removing debris, have the potential to be irritants.108 The alkaline nature of soap also negates the protective natural acidity of the skin, which in turn alters the balance of resident flora on the skin.109 Drying the skin by patting may cause less trauma,110,111 though if it leaves excess moisture on the skin this can lead to maceration and increased vulnerability to friction injuries.112 Many alternative cleansers have been marketed which address the shortcomings of soap and water, but little data exist to recommend any particular product at this time.113
Maintaining proper skin hydration is most often achieved through the use of emollients, which occlude the skin surface with a hydrophobic layer, and humectants, which attract and absorb water from their surroundings. Numerous formulations exist, many with additional surfactants and emulsifying agents, which vary somewhat in their effectiveness, greasiness, and potential for skin irritation. While the theoretical advantages of treating dry skin are well established,55,57,114 as with cleansers, few data exist to recommend any one hydrating product over another.115,116
Barrier products protect the skin, particularly in the setting of incontinence or in the presence of stomas, fistulas, or wounds. Many preparations consist of a lipid/water emulsion that forms a protective film over the skin. Newer barrier products incorporate a polymer which forms a thin semipermeable membrane over the skin.117 Many incorporate an antiseptic agent such as cetrimide or benzalkonium as well. Again, despite their widespread use and the proliferation of products, data on their effectiveness are scant.118
While data on individual agents are not very robust, there is evidence that a clear skin care protocol can benefit patients. Multiple authors have found instituting such protocols results in reduction in pressure sore incidence rates.119 Cole and Nesbitt120 found a reduction from 17.8% to 2% over a 3-year period, whereas Lyder et al.121 noted an 87% reduction in a nursing home setting. Based on the available data, the American Wound Ostomy and Continence Nurses Society122 produced a set of guidelines for skin care.
Incontinence
As noted previously, the relationship between urinary incontinence and pressure sore incidence is not clear, with limited evidence to suggest a causal relationship. Currently, the use of diapers or sanitary pads in conjunction with meticulous skin care is a reasonable option when compared to the risks associated with extended use of a urinary catheter.123
Fecal incontinence, on the other hand, has been shown to be a risk factor for pressure sores. At times fecal incontinence is due to factors which are not easily correctable: cognitive impairment, history of colorectal surgery, radiation proctopathy, inflammatory bowel disease, and various neurological or myogenic disorders of sphincter function are not easily correctable. The bladder and bowel dysfunction of SCI patients requires specific regimens and is generally managed by a specialist.124
However, many measures can be instituted to decrease the impact of fecal incontinence. Possibly the most common predisposing condition to fecal incontinence is fecal impaction, which is common in older adults and may lead to overflow incontinence.125 Conservative measures include diet modification and a wide variety of antimotility agents, including clonidine, cholestyramine, loperamide, codeine, diphenoxylate, and atropine.126,127 Diarrhea may be due to infection, which should be ruled out and treated prior to using antidiarrheal agents. If medical management is unsuccessful, surgery may be considered, ranging from attempts at sphincterplasty to elective colostomy when other options fail.128 Though patients and families are often reluctant to proceed with colostomy, there is evidence overall quality of life is improved in patients with severe fecal incontinence.129
Spasticity
Control of spasticity may not only reduce the risk of pressure sores, but may also improve patient reports of pain and ability to perform activities of daily living (ADL).130 However, it is worth noting that in some cases spasticity may increase stability in positioning, facilitate some transfers and ADLs, and prevent osteopenia.131,132 Taken as a whole, the impact of spasticity on quality of life is not straightforward, and should be considered before instituting treatment.
Physical therapy is the first step in treating spasticity and an important component of care in SCI patients in general.133 Pharmacologic treatment is the next step. Diazepam, baclofen, clonidine, tizanidine, gabapentin, and dantrolene are commonly used agents.134–137 Each has the potential for side-effects, including sedation, nausea, diarrhea, muscle weakness, and cognitive effects, and treatment must be tailored to the individual patient.137,138
Patients who cannot tolerate or do not respond to oral therapy may benefit from intrathecal administration of baclofen.136,139 As the drug directly accesses the central nervous system, systemic side-effects are minimized at the cost of the risks of surgery and possible mechanical complications with the pump. Injection with chemodenervation agents, including phenol, ethanol, and botulinum, can be effective.140,141 Reported complications include systemic side-effects, vascular complications, skin irritation, and tissue necrosis. Though the effects are temporary, long-term use of chemodenervation agents will result in denervation atrophy.
Surgical management of spasticity is an option when medical therapy is insufficient. Baclofen pump implantation is the most common surgical procedure for spasticity in individuals with SCI and has a history of success.137,142 In select, refractory cases, orthopedic and neurosurgical techniques may be of benefit, though such procedures are unlikely to have pressure sore prevention as their primary indication. Local tenotomy or tendon transfer has had mixed results in the treatment of spasticity.143 Rhizotomy has been complicated by both inadequate treatment of spasticity144 and severe atrophy,145 depending on the technique employed. Myelotomy and T-myelotomy involving direct sectioning of the spinal cord have been reported to be effective in several case series146,147 in patients who did not have hope of regaining motor function, though the benefits did decrease over time.148
Pressure relief
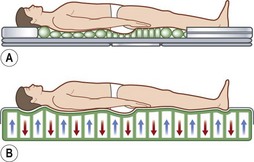
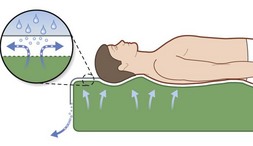
As befits its primary role in pressure sore pathogenesis, extensive efforts have been made in modulating the pressure through the use of various surfaces and products as well as protocols mandating patient repositioning. Though numerous support surfaces exist, most can be divided into two general categories. Constant low-pressure (CLP) devices distribute pressure over a large area and include devices such as static air, water, gel, bead, silicone, foam, and sheepskin supports (Fig. 16.7). Alternating-pressure (AP) devices vary the pressure under the patient, avoiding prolonged pressure over a single anatomic point149 (Fig. 16.7).
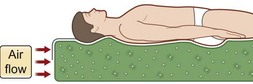
Two particular types of CLP device deserve special mention, as they are commonly used in the prevention and treatment of pressures sores. Low air loss (LAL) beds float the patient on air-filled cells through which warm air circulates (Fig. 16.8). The circulating air both equalizes pressure exerted on the patient and keeps the skin dry. Properly utilized, LAL surfaces exert less than 25 mmHg on any part of the body.150,151 Air-fluidized (AF) beds circulate warm air through fine ceramic beads, creating a unique support surface, while having a drying effect similar to LAL beds (Fig. 16.9). With proper use these beds exert less than 20 mmHg on the patient, but are expensive, heavy, and cumbersome.152
Within these classifications are a multitude of beds, mattresses, overlays, pads, and cushions, each with slightly different designs by different manufacturers. There is a daunting body of literature comparing various specific products to one another and with standard hospital beds. Evaluating this literature is challenging given the often small sample sizes, poor experimental design, and lack of generalizability common in these studies. Moreover, as new products are developed and old ones are phased out, many studies refer to products no longer in production or use. Despite these limitations, some general conclusions can be drawn from the literature.153
Considerable evidence exists that the use of a pressure-relieving overlay is superior to a standard bed in preventing pressure sores. Multiple studies have found decreased incidence and severity of pressure sores in high-risk patients with the use of constant low-pressure devices such as overlays154–156 or sheepskin157,158 when compared to a standard hospital foam mattress. Pooled analysis reveals a relative risk of 0.32.153 Data also support the use of AP devices when compared to standard mattresses. Both Andersen et al.156 and Sanada159 noted a significant reduction in pressure sore incidence with the use of active devices, with a pooled relative risk of 0.31.153 Though several studies have compared CLP and AP devices,156,160–162 no clear advantage has been identified, despite attempts at pooled analysis.153
LAL, like other CLP surfaces, has been shown to be effective at preventing pressure sores,163,164 with a relative risk estimated at 0.08.153 Comparisons with other CLP devices in regard to prevention are limited, and no clear advantage for either has been demonstrated.165,166 Likewise, though AF beds are perhaps the most effective pressure-relieving devices in widespread use, virtually no data on its role in prevention are available, perhaps because these expensive devices are reserved for treatment. Regardless of the type of mattress used, they lose their ability to disperse interface pressure as the head is elevated to 45° or higher.167 As such, patients should avoid prolonged periods of sitting while on these mattresses.
In addition to support surfaces, patient repositioning is often used in an attempt to prevent pressure sores, though the data are limited. Defloor et al.168 found a significant reduction in pressure sore incidence when turning every 4 hours combined with a specialized foam mattress was compared to every 2 hours on a regular mattress. Given the study design it is difficult to determine the relative contributions of the pressure relief surface and the turning regimen. The ideal interval and posture for repositioning are not clear given the current data.58,169 It bears mention that repositioning is not without its costs, both in terms of nursing time and effort, patient discomfort, and possible dislodgement of catheters and lines, and there is no evidence that more frequent repositioning reduces rates of pressure sores.
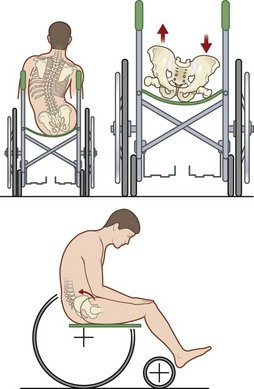
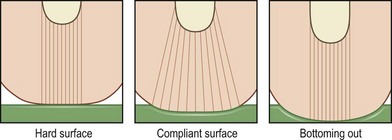
Cushions for wheelchairs filled with gel, foam, air, or water are available to relieve pressure. A typical wheelchair sling seat exerts a “hammocking” effect that can produce abnormal scoliotic posture and pelvic obliquity (Fig. 16.10). This in turn causes asymmetrical pressure on both trochanter and ischium that requires specialized cushions for prevention.170 Hip adduction and internal rotation of thighs, with consequent reduction in stability, are also seen in most paraplegics, who lack trunk or pelvic muscle innervation and tend to sit on their coccyx. Rigid-base cushions provide lumbar support and decrease ischial pressure by allowing wider weight distribution on the posterior thighs.170 A direct correlation between buttock–cushion interface has been noted171 (Fig. 16.11).
Houle172 and Souther et al.173 studied the effects of various wheelchair seats and found decreased pressures over the ischium, although none was below the capillary pressure benchmark. The authors recommend additional measures for pressure relief to prevent ulceration. Ragan et al.174 studied seat interface pressures on wheelchair cushions of various thicknesses. They found the highest subcutaneous stress concentrated within 2 cm of the ischial tuberosity. The subcutaneous pressures decreased with thicker cushions, with the maximum effectiveness obtained with an 8-cm cushion. Increasing the thickness beyond 8 cm did not give further benefit in reducing subcutaneous stress. As with pressure relief mattresses, no data exist to recommend a particular type of cushion over another at this time.153,160,175,176
The development of pressure consciousness by the patient is an essential part of pressure sore prevention.177 Pressure release maneuvers are reinforced to patients and should be performed at least every 15 minutes while the patient is seated.178
Nutrition
Multiple studies have examined the effect of nutritional supplementation on pressure sore prevention. Despite improvement in secondary measures such as caloric intake, body weight, and serum nutritional markers, most studies have failed to find reduction in pressure sore rates.179–181 Bourdel-Marchasson and Rondeau182 found a modest reduction in pressure sore rates when patients were given oral dietary supplementation. A more recent meta-analysis by Stratton et al.183 found a modest reduction in pressure sore rates in patients treated with enteral tube feeds, estimating that 19.25 patients would need to be given enteral nutritional support to prevent one pressure sore. Overall, the evidence that pressure sores can be prevented by nutritional supplementation, much less specific formulations or micronutrient additives, is limited.184
Nonsurgical management
Pressure relief
Pressure relief continues to be critical in the treatment of pressure sores. While more advanced LAL and AF surfaces would in theory be preferred in the treatment of established pressure sores, the literature is mixed on this point. Ferrell et al.185 found similar results when comparing LAL beds to foam mattresses. Ochs et al.186 in turn found AF beds to be superior to both standard overlays and LAL beds. On the other hand Branom and Rappl165 found LAL mattresses inferior to an advanced foam surface, while Economides et al.187 failed to find any superiority of an AF bed over a foam overlay. Overall there is insufficient evidence to recommend any particular pressure relief surface in the treatment of pressure sores, though given the theoretical advantages of improved pressure relief and moisture control afforded by LAL and AF surfaces, their use is not unreasonable.153,188
Spasticity
Spasticity should be addressed not only to improve patient positioning, weight distribution, and hygiene, but also to prevent tension on the healing wound, particularly if surgical intervention is planned.189,190 Spasticity treatment may be complicated by a reluctance to perform surgery or implant devices in a patient with an open wound, though this has been reported with favorable results.189 If pump implantation is not an option then temporary chemodenervation should be considered, with delayed pump placement once the wound is healed.73
Malnutrition
Malnutrition should be corrected and nutritional markers followed and trended. While many small studies have been published examining the effect on nutritional supplementation of the healing of pressure sores, many suffer from design flaws that limit their application. In 2003 Langer et al.184 felt the quality of the existing literature was inadequate to perform a meta-analysis and that no firm conclusions could be drawn. More recently both Heyman et al.191 and Frias Soriano et al.192 found improved wound healing with oral supplementation, but did not include controls in their studies. Van Anholt et al.193 and Lee et al.194 both found accelerated healing with the use of oral nutritional supplement in randomized control trials. There is currently little evidence to support supplementation of micronutrients such as vitamin C or zinc in the absence of a specified deficiency state.184,195
Infection
Osteomyelitis is a common complication of deep pressure sores and, as previously mentioned, often mandates operative therapy. Many authors rightly emphasize the importance of adequate debridement in the treatment of osteomyelitis.196 However, biopsy-directed antibiotic therapy is still an important adjunct to surgery.79 While traditional therapy calls for 6 weeks of intravenous antibiotic therapy, there is some evidence that shorter courses many be effective.87
Wound care
Debridement, regardless of the method used, should be the first step in the care of pressure sores. Only once the wound is thoroughly debrided can the full extent of the wound be examined and staged. Necrotic material impedes wound healing and acts as a nidus for infection. Traditional wet to dry dressing may debride effectively, but may remove healthy tissue and granulation in addition to necrotic tissue.197 They may also aerosolize bacteria from the wound.198 Allowing the wound to desiccate despite evidence that wound healing proceeds best in a moist environment is also counterproductive.199 Enzymatic debridement can be effective, though papain preparations are no longer available in the US. Biological debridement is enjoying something of a resurgence, using maggots that consume necrotic material while sparing living tissue. Currently no evidence suggests superiority of one agent over another.200 Ultimately, though many complementary methods of debridement are available, they are no substitute for proper sharp debridement.76
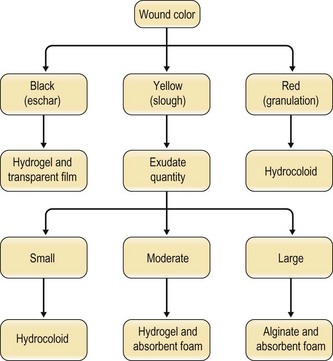
Selection among the many available dressings must be guided by the characteristics of the wound. Lionelli and Lawrence200 review the multitude of dressings available. In the context of pressure sores, occlusive films and hydrocolloids are frequently used on more shallow ulcerations while alginates find use in deeper, heavily exudating wounds (Fig. 16.12). It is worth noting that, even within a classification, individual products may differ widely in their capacity for absorption, occlusion, permeability, and cohesion.200 There exists little evidence to recommend a particular dressing in this context. When Bradley et al.201 performed a meta-analysis of the various dressings and topical agents used for pressure sore treatment, no significant differences were found.
As with dressings, numerous topical agents are used in the hopes of improving wound healing. Antiseptic solutions are commonly used in hopes of reducing the bacterial burden of a wound. However, while mafenide,202
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree