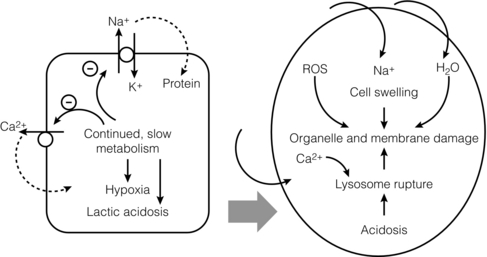
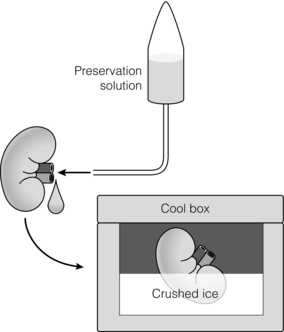
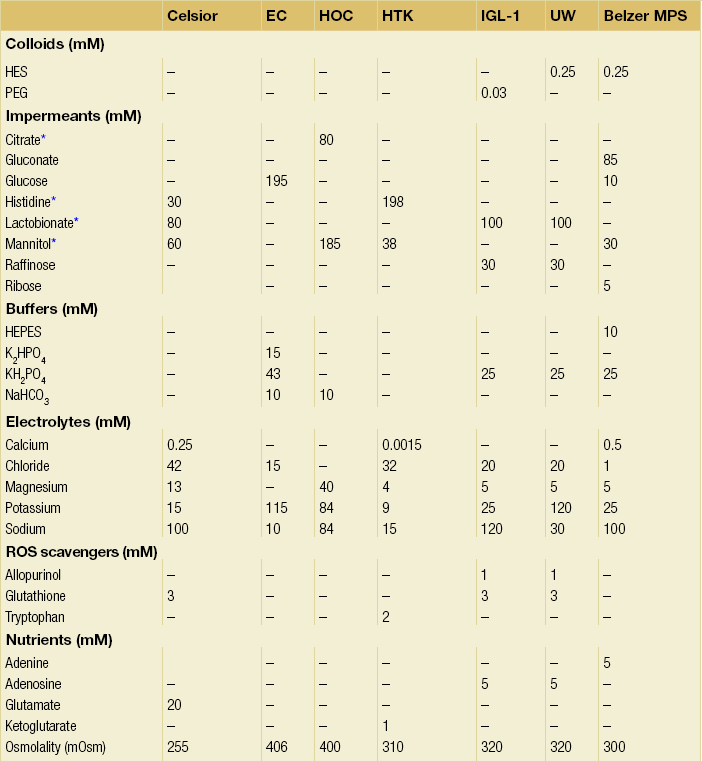
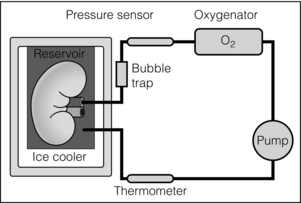
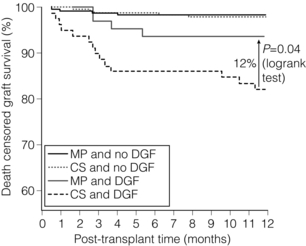
6 Despite efforts to increase the number of organs available from the classical donor type (the deceased, heart-beating donors, or donation after brain death (DBD)), transplant waiting lists remain long and in some cases ever-extending. Donation after circulatory death (DCD) has therefore been increasingly used in some countries,1 as have expanded criteria donors (ECDs).2 ECDs are, by their definition, associated with reduced graft survival and include all deceased donors over 60 years old, or aged 50–59 years, with at least two of the following conditions: cerebrovascular cause of death, serum creatinine over 1.5 mg/dL, hypertension.3 Donor comorbidities are associated with a set of problems that need to be addressed by the preservation method in order to improve the outcomes from these organs. Organs from DCD may be categorised using the Maastricht Criteria (see Table 6.1). They suffer a longer period of warm ischaemia after the withdrawal of supportive treatment in the case of Maastricht Category III donors and an even longer warm ischaemic time (WIT) in the case of the so-called ‘uncontrolled’ donors of Maastricht Categories I, II and IV.4 Kidneys from DCD have a higher risk of delayed graft function (DGF) and primary non-function (PNF),5 and livers a higher risk of biliary complications and re-transplantation.6 The tissue injuries associated with this long first warm ischaemic period have prompted the development of new organ preservation techniques to prevent the extension of this damage and to resuscitate the organ if possible. Table 6.1 Maastricht categories of deceased donors after cardiac death The spectrum of donors contributing to the DBD organ pool has also changed over the last 30 years, with an increasing number of older individuals becoming donors of this type.7 Cerebral injury followed by brain death sets in motion a number of inflammatory processes that can directly injure organs, but also prime them to undergo further damage at reperfusion.8,9 Kidneys from DBD start to undergo a process of damage following ischaemia of the brain; urinary sodium excretion increases, renal tubules undergo necrosis and the arterial intima undergoes fibrous proliferation.10 There is evidence that brain death increases the immunogenicity of transplanted organs,11 associated with a higher rejection rate when compared with organs from live donors.12 If an organ remains at body temperature after circulatory arrest, tissue metabolism will continue at a normal rate for some time. Without a normal circulation to remove metabolites and deliver nutrients and oxygen, catabolic enzymes will be activated and anaerobic metabolism will lead to cellular acidosis and, ultimately, cell death. A simple method to reduce metabolic rate and protect against warm ischaemia is to cool the organ. Metabolism does not cease at low temperatures though, and it has been known for some time that there is a continued, but greatly reduced, oxygen and nutrient requirement during the cold preservation phase.13 Tissue cooling and hypoxia reduce ATP synthesis, resulting in reduced Na/K-pump activity, with sodium passively entering the cell unopposed. Water subsequently follows the osmotic gradient into the cell, causing cell swelling and lysis. The swelling of endothelial and perivascular cells can cause resistance to blood flow and vessel blockage. Ongoing metabolism in hypoxic conditions leads to an accumulation of lactic acid, causing lysosomal rupture, organelle and membrane damage, and subsequently cell death.14 See Figure 6.1. Figure 6.1 Negative effects of cold ischaemia. Continued but slowed metabolism results in hypoxia, acidosis, reduced ATP synthesis, and activity of sodium and calcium pumps. Resultant sodium influx with water leads to cell swelling. ROS (reactive oxygen species), along with acidosis and lysosomal enzymes, damage cellular organelles and membranes. During re-implantation of the organ a second period of warm ischaemia is suffered while vascular anastomoses are fashioned and tissue perfusion is reconstituted. The pre-existing damage accrued in the first warm period and the cold preservation stage now becomes evident. The oxygen-rich blood allows the formation of reactive oxygen species (ROS) that are extremely damaging to cellular organelles and membranes, the so-called ‘ischaemia–reperfusion injury’ or IRI15 (see Chapter 3 for more details). Surface cooling was first tested as a method to reduce the temperature of renal autografts, with Calne et al. demonstrating that surface cooling could be effective in animals.16 Surface cooling is slow to achieve low temperatures within the tissues, so a move to perfusion cooling through the abdominal arteries was taken. Initially autologous blood was used, although this was not much quicker, due to increased blood viscosity at lower temperatures. Continuous cold perfusion of the graft became the norm in kidney transplantation until the development of better preservation solutions for static cold storage (SCS) that demonstrated equivalent results to machine perfusion.17 This simpler method requires a rapid flush-out of blood in situ using the preservation solution and after retrieval the organ is submerged in a sterile bag of the same solution. This bag is then buried in ice in a cool box. SCS is relatively cheap and requires the minimum of input from the retrieval or implant team during the preservation period. See Figure 6.2. Figure 6.2 For static cold storage, kidneys are flushed through the renal artery with preservation solution after removal from the donor. Preservation fluid is run until the effluent from the renal vein is clear. The kidney is submerged in the preservation fluid in a sealed bag, which is then packed in a cool box surrounded by crushed ice. Given the mechanisms of damage mentioned above, preservation fluids were specifically designed to counteract each harmful pathway and preserve the physiological and biochemical conditions of the organ. Of particular importance are the impermeant molecules that prevent the movement of electrolytes and water across the cell membrane. They must remain within the vascular space and/or the interstitium to be effective. Hypertonic solutions were first tested in this capacity. In 1969, Collins et al. developed the first SCS solutions, testing four similar fluids based upon intracellular electrolyte composition, with a high concentration of glucose as an impermeant. The group showed that SCS could be used to preserve kidneys for up to 30 hours.18 The original Collins solution was later modified by the Eurotransplant Foundation to Eurocollins solution (EC) by the removal of magnesium. A number of impermeant molecules have subsequently been tested, including sucrose,19,20 mannitol21 and citrate.22,23 Colloids such as starches are also very effective impermeant molecules, as they have a large molecular weight and are retained in the vascular compartment. Molecular weight determines the success of extracellular impermeants in preservation solutions, with larger molecules being more successful as impermeant components.24 In the past decades a number of preservation solutions have therefore been developed to counteract the detrimental effects of the retrieval process, graft cooling and reperfusion. They specifically target the biochemical and structural changes that occur during this process, yet vary considerably in the type and concentration of their constituents. A few examples of the most commonly used fluids today are described below (details are given in Table 6.2); more organ-specific considerations will be given in the appropriate sections. Table 6.2 Composition of preservation solutions for static cold storage* and hypothermic machine perfusion. *Citrate, histidine and lactobionate also act as buffers. Histidine, lactobionate and mannitol also act as ROS scavengers. University of Wisconsin solution (UW, Viaspan®, Bristol-Meyers Squibb, also supplied as SPS-1®, Organ Recovery Systems and Belzer UW Cold Storage Solution®, Bridge to Life) was elegantly designed by Belzer and Southard in the 1980s to counteract the well-known effects of organ retrieval and hypothermic preservation, and showed good results in kidney, liver and pancreas preservation.25–27 UW has an intracellular-type electrolyte composition, with 120 mM potassium and 30 mM sodium. It contains three impermeant molecules, lactobionate and raffinose, with the colloid hydroxyethyl starch (HES), which in combination are very effective at preventing tissue oedema. Lactobionate, a large anion, also acts as a buffer and free radical scavenger. Glutathione acts a free radical scavenger through its preferential oxidisation, while adenosine is a nutrient precursor. Allopurinol included in UW inhibits xanthine oxidase and hence free radical formation. The addition of the ATP precursor adenosine provides for ongoing metabolism. Some studies have moved on to alter the basic composition of UW slightly to demonstrate that the Na/K ratio could be reversed,34 and dextran could be substituted for HES.35 Histidine–tryptophan–ketoglutarate (HTK, Custodiol®, Dr Franz Kohler Chemie GMBH) was originally used as a cardioplegic solution, but has since been tested in the preservation of kidney, liver and pancreas.29,30,33 It has been used in the preservation of increasing numbers of kidneys since the early 1990s.36 HTK contains a much lower concentration of potassium than UW (9 mM). There are several other differences in the composition; the amino acid histidine is the main impermeant molecule, assisted by mannitol. HTK also does not contain a colloid, such as the HES used in UW, and is therefore much less viscous.37 Histidine also acts as a buffer in the solution while tryptophan and ketoglutarate are free radical scavengers and metabolic precursors, respectively. To achieve the maximal buffering capacity, relatively large flush volumes are recommended (10–15 litres). Celsior (Celsior®, Genzyme) was developed initially for cardiac allograft preservation in the early 1990s; it has since proven to be of use in the preservation of kidneys, liver and pancreas.31,32,38 Unlike UW it has a high sodium content (100 mM) and low potassium content (15 mM), and is relatively low in viscosity. Celsior incorporates lactobionate as an impermeant, which acts through both its molecular weight and negative charge. Histidine is the main buffer and reduced glutathione acts as a scavenger of ROS. It also contains mannitol as a second impermeant and free radical scavenger, along with reduced glutathione as an antioxidant. The use of polyethylene glycol (PEG) in preservation solutions was prompted by the high viscosity associated with HES, and its tendency to cause red cell aggregation in animal experimental models.37 This effect has not been demonstrated in humans. PEG has been attributed with the potential to prevent immune interactions by binding to the surface of cells.39 Institut George Lopez-1 Solution (IGL-1®, Institut Georges Lopez) is similar to UW in the inclusion of raffinose, lactobionate and potassium phosphate; however, it has PEG instead of HES as an impermeant colloid. It has shown potential in the preservation of kidney, liver, pancreas and small bowel.40–44 When kidney transplantation programmes were first established, hypothermic machine perfusion (HMP) was used to preserve kidneys and demonstrated good results.45,46 With the development of better preservation solutions that demonstrated equivalent results at reduced immediate costs, SCS became the prevalent method of storage. Although the new generations of pumps for HMP are lighter and smaller than those used 40 years ago, the principle has not changed; chilled preservation fluid is pumped in a pulsatile or continuous fashion through the renal artery in a recirculating unit. Complete washout of blood and uniform organ cooling is achieved. See Figure 6.3. Figure 6.3 Hypothermic machine perfusion devices maintain the stored kidney in a bath of cooled preservation solution surrounded by ice. The same solution is withdrawn from the bath and pumped (via an oxygenator in some cases) through the renal artery of the kidney. Temperature and flow dynamics can be monitored. A considerable difficulty associated with hypothermic perfusion is that of tissue oedema due to the hydrostatic pressures. In order to overcome this problem a perfusion fluid with strong oncotic pressure is required to retain water in the vascular space. Initially human albumin solution was used for this purpose, and later human cryoprecipitated plasma, allowing the first successful machine preservation of a human kidney for 17 hours.46 The synthetic perfusate Belzer machine perfusion solution (Belzer MPS, KPS-1®, Lifeline Scientific Inc.) was later developed and has since become the most widely used machine perfusion fluid. Attempts to improve upon Belzer MPS through the addition of metabolic substrates, antioxidants and vasodilators (Vasosol®, Procent Technologies LLC) have shown the potential to further reduce DGF following kidney preservation.50 Static cold preservation remains the most commonly used method for preservation of kidneys from deceased donors worldwide, whether they are DCD or DBD. Data from the international Collaborative Transplant Study (CTS) reveal that during the period 1990–2005 SCS was used for approximately 97–98% of all kidneys, with machine preservation making up the remaining 2–3%.36 UW solution was established as the gold standard for kidney preservation in the early 1990s following a large international RCT that demonstrated its superiority over the previous gold standard, Eurocollins.28 This study demonstrated that preservation in UW could deliver reduced rates of DGF, a more rapid fall in serum creatinine and improved graft survival. UW therefore replaced Eurocollins as the dominant solution over the coming years, increasing in use from approximately 46% of deceased donor kidneys in 1990 to 70% by 2002.36 In the early 1990s the HTK solution was also shown in a large international RCT to be superior to Eurocollins by reducing DGF,29 although no impact on graft survival was seen in this study. In the same international RCT, HTK was shown to be equivalent to UW;29 however, the debate has continued concerning the relative benefits of UW over HTK, with registry analysis demonstrating that HTK was associated with an increased risk of graft loss and PNF.36,51 The rate of DGF was found to be the same between the two solutions. As cold ischaemic time (CIT) increases, both HTK and UW show a mild increase in graft loss; however, at CIT longer than 24 hours there is a suggestion of an advantage of UW over HTK.36 More recently, other solutions have been used for the preservation of kidneys and tested against UW. Celsior has been compared to UW in three large multicentre RCTs, demonstrating comparable rates of DGF and graft survival.38,52,53 IGL-1 solution has been compared to UW in one small cohort study demonstrating comparable rates of DGF and graft survival.54 Serum creatinine levels were lower for IGL-1 from days 7 to 14 postoperatively in this study. Overall it would appear that there is good evidence from RCTs that Celsior, HTK and UW are of equal quality for the preservation of standard quality kidneys. There are some concerns raised, however, about the use of HTK, but these arise from retrospective, non-randomised data. In multivariate analysis HMP was found to be independently associated with a significantly reduced risk of DGF. The duration of DGF, when it occurred, was also shorter if following HMP. One-year and 3-year graft survival was also significantly higher following HMP compared to SCS (94% vs. 90% and 91% vs. 87%). In those donor kidneys that developed DGF, 1-year graft survival was 12% lower if cold stored than if machine perfused.47,55 See Figure 6.4. Figure 6.4 Results from Moers et al.47 showed that hypothermic machine perfusion was particularly effective in improving graft survival for kidneys with delayed graft function. Delayed graft function following static cold storage was associated with the worst 1-year graft survival. As the more standard donor type internationally, DBD kidneys have been the focus of most preservation trials, if not a major part of studies in combination with DCD. The preservation solutions for SCS mentioned above were tested in trials largely relying on DBD kidneys, except for the European multicentre comparison of UW with Eurocollins,28 and HTK with Eurocollins,29 which used DBD kidneys alone. Despite the already low rate of DGF experienced by kidneys from DBD, the European machine preservation trial discussed above showed this rate could be further reduced from 21% to 16%, when compared to SCS.47 The use of kidneys from controlled DCD has risen in response to the falling number of suitable DBD kidneys and comparable long-term outcomes between the two donor types have been demonstrated.1,56 However, the injury suffered by kidneys from the two donor types differs and it is therefore reasonable to speculate that the best preservation method for each donor type may be different. Kidneys from all four Maastricht classes of DCD donors are used to varying degrees worldwide, but most commonly it is those from classes II and III. Various protocols are in place for the procurement of kidneys from Maastricht class III donors, but by and large the ultimate preservation method used has been SCS in a preservation fluid, as described above for DBD kidneys. A large meta-analysis of available data on DCD and DBD kidneys shows that DCD kidneys are at a much higher risk of DGF (odds ratio (OR) 3.64) and PNF (OR 2.43). This is believed to be a result of the variable warm ischaemia suffered by the organ following the withdrawal of life-supporting treatment, or pre-hospital cardiac arrest.56 The potential benefit from a reduction in DGF and PNF is therefore greater for DCD kidneys. It has been speculated that DCD kidneys would receive greater benefit from hypothermic machine perfusion than DBD kidneys. Two recent RCTs have examined the effects of this preservation modality in DCD kidneys. As part of the European multicentre RCT described above, a separately paired RCT was conducted using DCD kidneys.57 This study showed that the rate of DGF could be decreased in this population from approximately 70% to 54% compared to static cold preservation. One-year graft survival was similar in both groups (94% vs. 95%), as was creatinine clearance at 1 year, although it was better for the first month postoperatively in the machine perfusion group. In contrast to this, the UK multicentre RCT found a similar rate of DGF in the two groups (56% vs. 58%), graft survival at 1 year (93% vs. 98%) and estimated glomerular filtration rate (eGFR) at 1 year.48 This difference may possibly be explained by the lower rate of DGF in the control arm of the UK trial. The outcomes may also have been influenced by the period of SCS used for kidneys before machine perfusion in the UK trial, whereas in the European trial machine perfusion was used from the point of retrieval until implantation. An experimental model of DCD kidney preservation is supportive of this theory, finding that HMP after a period of static preservation was not beneficial compared to static preservation alone.58 Kidneys stored statically to begin with had improved renal resistance during the course of the subsequent HMP; however, the renal resistance started at a much higher level than those that went straight on to a pump. Four hours of HMP could not bring the renal resistance back in line with the kidneys that were pumped from the beginning.58 In countries without a legal definition of brain death or an overall shortage of organs, uncontrolled DCD are an important source of kidney allografts. Using strict selection criteria, one centre in Madrid, Spain, has successfully transplanted kidneys from Maastricht categories I, II and IV.59–61 Following irreversible cardiac arrest in suitable donors, a femoral arterial and venous catheter is inserted, allowing the maintenance of grafts by extracorporeal membrane oxygenation (ECMO), sometimes called normothermic regional perfusion (NRP) in this context. ECMO systems are widely used in intensive care units to support patients with cardiorespiratory failure. The system circulates the patient’s blood through an oxygenator and warmer. In the setting of organ donation it theoretically allows the continued circulation of warm, oxygenated blood through the abdominal organs after cardiac arrest. A double balloon is used to occlude the aorta and prevent recirculation through the brain or heart. This stop-gap allows judicial review and contact with the family while preserving the kidneys for a maximum period of 4 hours. Standard retrieval can then be performed. This method has demonstrated good results, with 1- and 5-year graft survival of approximately 85% and 83%, respectively, compared to 87% and 84% for DBD kidneys at the same institution during the same time period.61 These kidneys undergo a variable amount of warm ischaemia of up to 2 hours, and the discard rate for retrieved kidneys is therefore high, at approximately 33%. The DGF rate ranges from 68% to 80% and the PNF rate is 6%.59,60 A series of kidneys from Maastricht I and II DCD in Paris, France, with a longer WIT of up to 150 minutes, demonstrated a higher discard rate (43%) and higher DGF rate (92%), but lower PNF rate (2%).62 Graft survival was 89% at 6 months. In a small series of Maastricht II and IV donors at one centre in Barcelona, Spain, normothermic ECMO was compared with hypothermic ECMO and with in situ cold perfusion by Eurocollins.63 The normothermic ECMO group had lower rates of DGF compared to the other groups and less PNF than the in situ group. By definition, kidneys from ECDs have worse graft survival than standard criteria donors (SCDs).64 The potential to improve upon their outcomes through better organ preservation and resuscitation is therefore greater. As part of the European machine perfusion trial, described above, a paired RCT was completed using ECD kidneys.65 This study showed that the rate of DGF could be decreased in this population from approximately 30% to 22% compared to SCS. One-year graft survival was better following HMP (92% vs. 80%), as was creatinine clearance at 1 year. Particularly striking in this study was the poor 1-year survival of kidneys that had DGF following SCS compared to HMP (41% vs. 85%); there was no significant difference in survival between kidneys with and without DGF following HMP. Rates of PNF were also reduced from 12% to 3% following HMP. Interestingly, in this study the rate of DGF was not significantly different comparing ECD with SCD kidneys. A smaller non-randomised controlled trial in ECD showed a more drastic reduction in DGF rate with HMP compared to SCS (9% vs. 32%).66 Renal resistance during HMP is often used to assess kidney viability and predict both short- and long-term outcomes. The European machine perfusion trial transplanted 302 kidneys with blinding of the surgeons to the machine perfusion parameters. High renal resistance was found to be a predictor of DGF and also 1-year graft loss, but the predictive value was low.57 Given the multifactorial causation of DGF, renal resistance cannot as yet be used to predict outcome on its own. Several biomarkers measured during HMP have also been implicated in the outcome of kidneys after transplantation, but until recently all evidence was retrospective and subject to selection bias. Six of these biomarkers were recently assessed during the Eurotransplant machine perfusion trial.67 These included alanine-aminopeptidase (Ala-AP), aspartate aminotransferase (AST), glutathione-S-transferase (GST), heart-type fatty acid binding protein (H-FABP), lactate dehydrogenase (LDH) and N-acetyl-β-D-glucosamine (NAG). Multivariate analysis of perfusates at the end of the preservation period showed that GST, H-FABP and NAG were moderate, independent predictors of DGF.67 None of the biomarkers were independently associated with PNF or decreased graft survival and should not be used as grounds to discard a kidney. None of the biomarkers correlated with renal resistance or cold ischaemic time, suggesting that they represent a different aspect of tissue injury. Future developments in kidney preservation will aim to repair damaged organs by providing mechanisms to support metabolism and remove waste products, counteracting the effects of ischaemia–reperfusion injury. New preservation fluids may allow static storage at normothermic or subnormothermic temperatures and this has been demonstrated in animal studies. AQIX, a non-phosphate-buffered solution, has been used for short-term, subnormothermic, static preservation in a porcine experimental model.68 Improved outcomes following static cold preservation may also be possible with the development of new solutions. Polysol, a low-viscosity solution, has been tested in porcine autotransplant models, showing improved renal blood flow and tissue oxygenation compared to UW69 and improved creatinine clearance compared to HTK.70 Delivery of oxygen to the graft during the preservation period has been the focus of several studies and various methods, other than the delivery of oxygenated whole blood, have proven to be promising in animal and experimental studies. Persufflation of gaseous oxygen directly through the vasculature of the kidney has been tested as a rescue therapy for ischaemically damaged kidneys. Filtered and humidified gaseous oxygen can be administered through the renal vein and allowed to escape from the surface of the kidney through fine pin-pricks made with a microvascular needle. In animal studies, autotransplanted kidneys displayed better creatinine clearance following oxygen persufflation, rather than cold static or perfusion preservation alone.71,72 The Groningen and Poitiers groups in collaboration have tested an oxygenated perfusate to preserve kidneys during HMP, and this has been tested in animal models of DCD and in autotransplant models.73,74 Membrane oxygenators within machine perfusion systems have been shown to promote ATP synthesis, even following 30 minutes of warm ischaemia.75 Oxygenation of machine perfusates with 100% oxygen to achieve very high oxygen partial pressure has also been tested as an adjunct to machine perfusion, and can recondition kidneys previously stored for 18 hours by SCS.76 On reperfusion, kidneys previously recirculated with oxygenated perfusate showed improved urine production and creatinine clearance. Oxygen could also be delivered to tissues via soluble oxygen carriers (such as Lifor; see above). The respiratory pigment Hemarina-M101, a large haemoglobin molecule derived from marine invertebrates, has been used to supplement standard preservation fluids in a static cold preservation animal study.77 Hemarina-M101 improved metabolic activity of preserved cell lines, increased ATP levels, improved creatinine clearance and reduced graft fibrosis following transplantation of preserved whole organs.77 Another possible strategy for the in situ protection of organs is the use of ECMO, alternatively known as NRP in the context of organ retrieval. While this technique is used for uncontrolled DCD in some countries (Spain and France, for example), it has not been widely tested in controlled DCD. ECMO provides a possible method by which the acidosis and low venous oxygen levels present after the withdrawal of life-supporting treatment could be reversed in controlled DCD. Following a decision to withdraw treatment on the ICU, family consent for donation is sought, and if agreed a femoral catheter is inserted and supportive treatment withdrawn. Five minutes after cardiac arrest the aortic occlusion balloon is inflated and NRP is initiated. In a case series from Michigan, USA, this method has provided low rates of DGF (8%) and PNF (0%) for Maastricht III DCD.78 Another group from North Carolina, USA, transplanted a series of kidneys following subnormothermic (22°C) regional perfusion after controlled DCD.79 DGF rates were higher in this study (57%). One-year graft survival was 87%. A large case series from Taipei, Taiwan, demonstrated that NRP for kidneys from Maastricht category II, III and IV donors could result in excellent 5-year graft survival rates of approximately 88%.80 This survival rate is comparable to that for live donor kidneys (89%) and DBD kidneys (83%) transplanted at the same institution during the same time period. The next logical step would seem to be the combination of normothermic preservation with continuous or pulsatile perfusion to remove metabolites and maintain constant intravascular conditions. Synthetic fluids could possibly be combined with normothermic perfusion. Lifor, a solution incorporating a non-protein oxygen carrier, has been tested for short-term in situ normothermic preservation81 and in a porcine model of subnormothermic renal perfusion.82 Lifor demonstrated a better preservation of renal function81 and reduced renal resistance compared to UW.82 A haemoglobin-supplemented fluid allowed the resuscitation of kidneys despite 2 hours of warm ischaemia, providing life-sustaining function.83 Another option would be to use blood itself and, in an animal study, normothermic perfusion with heparinised whole blood has shown preservation of renal function during 6 hours of perfusion, despite following up to 40 minutes of warm ischaemia.84 The first published case was that of an ECD declined by six UK centres before being accepted in Leicester. One kidney was perfused at normothermic temperatures with plasma-free, red-cell-based solution for 35 minutes prior to transplantation, while the other kidney was stored by static cold preservation alone. Both kidneys had acute tubular necrosis and rejection on protocol biopsy at 1 week; however, the recipient of the reperfused kidney had primary function, while the recipient of the static cold preserved kidney required dialysis for a further 4 weeks. The Leicester group have moved on to use this method of pre-implantation, normothermic recirculation for a case series of 16 transplants, with promising results so far.87 One option in the prevention of ischaemia–reperfusion injury may be ‘immune camouflage’ whereby selected polymers interact with allograft cell membranes to reduce immune cell invasion of the graft. PEG, a constituent of IGL-1 solution, is one such polymer. Animal studies have suggested that plasma with PEG may have better static preservation effects than IGL-1 or UW with less immune cell infiltration and improved graft survival.88
Preservation and perfusion of abdominal organs for transplantation
Introduction
Category
Description
I
Dead on arrival at the hospital
II
Unsuccessful resuscitation at the hospital
III
Withdrawal of supportive treatment
IV
Cardiac arrest following establishment of brain death
Development of preservation techniques
Static cold storage
University of Wisconsin solution
Histidine–tryptophan–ketoglutarate solution
Celsior solution
Institut-Georges-Lopez-1 solution
Hypothermic machine preservation
Kidney
Donation after brain death
Controlled donation after circulatory death
Uncontrolled donation after circulatory death
Expanded criteria donors
New developments and the future
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






