Key points
- •
Three clinical and histological variations.
- •
Differentiation from nodules.
- •
Eight most commonly used dermal fillers.
- •
Treatment options: intralesional steroids.
- •
No surgical excision of granulomas.
Introduction
Granuloma formation following implantation of injectable dermal fillers is a rare complication, with incidences ranging from 0.02–1%. Foreign body granulomas (FBG) can occur several months or years after injection at all implantation sites. Without treatment, they may grow to the size of beans, remain virtually unchanged for some years, but then resolve spontaneously.
Three clinical and histological types of foreign body granulomas after fillers can be distinguished:
1. Cystic (or inflammatory, palisading, necrobiotic, collagenolytic) granulomas are mainly caused by injected biological gels such as collagens and hyaluronic acids. Their clinical signs are fluctuation (sterile abscess), extreme redness, and induration. Cystic granulomas are small and superficial, occur within the first year and generally disappear spontaneously within the year after their appearance. They are surrounded by a significant number of giant cells.
2. Edematous (or lipogranuloma) granulomas are caused by artificial fluids such as silicone and polyacrylamides. They appear suddenly with extensive swelling, years after injection and are surrounded and infiltrated by mononuclear and inflammatory cells.
3. Sclerosing (or sarcoidal, xanthelasmic, lipogranuloma) granulomas are caused by particulate injectables composed of polymethylmethacrylate (PMMA), polylactic acid (PLA), polyhydroxy-ethylmethacrylate (pHEMA), calcium-hydroxylapatite (Ca-HA) or dextran microspheres. Generally, sclerosing granulomas occur 6 months to 3 years after implantation and are visible, often bluish confined nodules. Histologically, the implant is infiltrated by many macrophages and giant cells, fibroblasts and collagen fibers, but few inflammatory cells.
Permanent implants are not necessarily characterized by a higher rate of granulomas per se than temporary implants. However, their clinical appearance is more pronounced and their persistence longer if not treated adequately. The treatment of choice is intralesional corticosteroid injections as for keloids. Surgical excision of true granulomas is generally contraindicated since their borders with the surrounding tissue are seldom defined and recurrence is therefore anticipated. In the sclerosing type, surgical excision with removal of the visible nodule may be necessary if intralesional treatments fail (e.g. a lump on a lip).
Background
The injectable dermal filler market has undergone dynamic growth since the public became aware of non-surgical approaches leading to a wrinkle-free skin. Resorbable and non-resorbable materials have been made injectable and are introduced beneath wrinkles and in skin depressions. In general, all injectable substances which exert a positive effect may also be expected to cause side effects. All current dermal fillers are associated with adverse effects ( ). Biological substances such as collagen or hyaluronic acids may cause lumps, allergies, long lasting redness, scarring, sterile abscesses, and eventually early foreign body granulomas. Longer lasting artificial substances, such as many polymers used in medicine, may cause lumps, persistent redness, and late granulomas.
The incidence of graulomas appears to vary according to the chemical nature of the injectable, its surface structure and properties and the content of its impurities, but not its primary biocompatibility or the volume injected. A strong histological foreign body reaction within the first months following injection is not an indicator for the increased possibility of late granulomas. The trigger for the sudden occurrence of a granuloma is not known. Anecdotal reports suggest this may be due to a systemic bacterial and/or viral infection in the months prior to the onset of a granuloma or due to localized trauma. Only experience and education gained by physicians and patients in the future will provide a realistic picture of this new field of injectables. The current climate is one of enthusiasm and negligence, warnings and hypotheses, widespread happiness, but also some disastrous results. Carefully controlled studies and accurate reporting of adverse events will be necessary to overcome anecdotal reports of problems.
Description of a foreign body granuloma
Granuloma is a compound of the Latin ‘granulum’ (little grain) and the Greek ‘onkos’ (tumor or nodule). In histopathology, it describes a granulomatous tissue reaction to bacteria (tuberculosis, leprosy, dental granuloma), fungi ( Actinomyces ), eggs of dermal parasites, unknown stimuli (lymphogranulomatosis, erythema nodosum, granuloma annulare, granulomatous lymphoma, pyogenic and eosinophilic granuloma), or foreign bodies (spines or stings, sutures, fat necrosis, surgical powder, tattoos, injectable filler substances). It is the body’s attempt to get rid of the intruded material. Histologically, granulomas consist of an inflammatory infiltrate made of histiocytes and epitheloid cells. They differ largely by the proportion and arrangement of lymphocytes, plasma cells, neutrophils, eosinophils, and multinucleated giant cells, as well as the amount of polymorphous exudates and sometimes the presence of necrosis.
Clinical appearance of foreign body granulomas
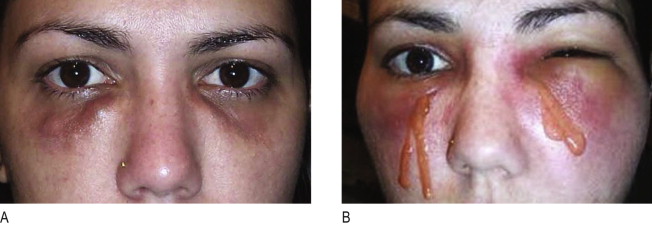
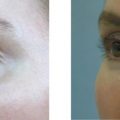
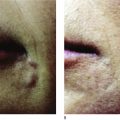
Irrespective of its histological description, a true granuloma is and remains a clinical diagnosis ( ). It can develop slowly or rapidly in certain patients after the injection of any dermal filler such as collagen, hyaluronic acid, silicone, polyacrylamides, and particulate polymers. It occurs significantly less often after implantation of microspheres ( Figure12.1A ) with smooth surfaces (Artecoll®, New-Fill™/Sculptra™) than after implantation of particles with irregular or edged surfaces (Bioplastique®, Dermalive®) ( Figure 12.1B ). Its appearance is less dramatic after resorbable implants (collagen, hyaluronic acid) ( Figure 12.2A ) than after long-lasting fluidal implants (polyacrylamide gel, silicone fluid) ( Figure 12.2B ).
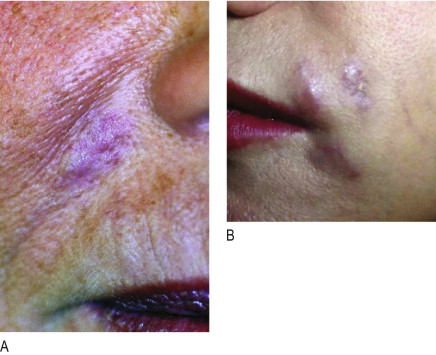
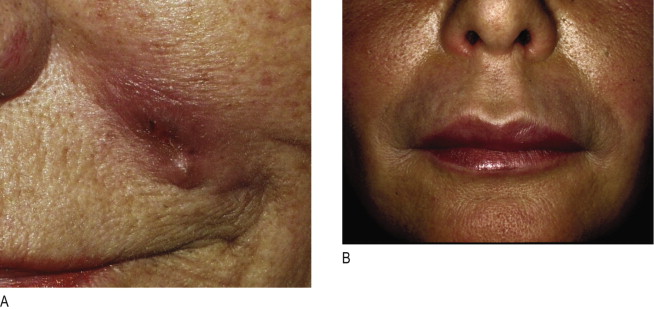
The time between injection and the appearance of a granuloma is usually 6–24 months. However, sudden occurrence of granulomas has been described up to 10 years following implantation ( , ). Some granulomas have developed only after the second or third implantation and others have developed years after the material had been absorbed ( ). True granulomas increase in size over time. Congested dermal capillaries widen and give the lump a bluish appearance. If not treated intralesionally with corticosteroids, they may increase to the size of a pea or a bean, remain unchanged in their clinical appearance, but resolve spontaneously after several years ( , , ).
Granulomas are rare in their occurrence and range from 1 : 100 to 1 : 5000 patients (1.0–0.02%) after injection according to surface structure and chemistry of various dermal filler substances ( Table 12.1 ). In general, granulomas are of non-allergenic origin and therefore a typical granuloma ( Figure 12.1A ) is not a late type IV allergic granulomatous reaction ( ). The unequivocal diagnosis of a granuloma is based both on histological evidence but largely on clinical appearance ( ).
| Product | References | Data collection | FBG / #Patients | CALCULATED FBG RATE (%) | |
|---|---|---|---|---|---|
| Individual | Overall | ||||
| Collagen gel | Cooperman | 1975–1984 | 15 * in 5109 | 0.3 | |
| Charriere | 1986–1988 | 8 * in 656 | 1.2 | ∼0.34 | |
| Castrow | 1981–1982 | 21 * in ∼7000 | 0.3 | ||
| Hanke | 1981 – 1989 | * in 470,000 | 0.04 | 0.04 | |
| Hyaluronic acid gel | Lowe | 1996–2000 | 3 * in 709 | 0.4 | |
| Andre’ | 1997–2001 | 18 * in 4320 | 0.4 | ∼0.4 | |
| Friedman | 1999 | some in 144,000 | 0.07 | ||
| Friedman | 2000 | rare in 262,000 | 0.02 | 0.04 | |
| PMMA microspheres | Lemperle (Arteplast ® ) | 1989–1993 | 15 in 587 | 2.5 | ∼1.51 |
| Gauthier (Arteplast ® ) | 1993–1994 | 9 in ∼ 1000 | 0.9 | ||
| Gauthier (Artecoll ® ) | 1995–1999 | 3 in ∼2000 | 0.15 | ||
| Lemperle (Artecoll ® ) | 1994–1998 | 7 in ∼3500 | 0.24 | ||
| Dansereau ** | 1998–2005 | 2 in ∼ 2000 | 0.10 | ∼ 0.16 | |
| Canderm Canada ** | 1998 – 2005 | 14 in 50,000 | 0.03 | 0.02 | |
| Hafod China ** | 2002 – 2005 | 2 in 30,000 | 0.01 | ||
| TRM Korea ** | 1996 – 2005 | 9 in 60,000 | 0.01 | ||
| Ca-apatite microspheres | Jansen | 2002–2004 | > 1 in 609 | 0.16 | |
| BioForm Medical ** | 2002 – 2005 | > 3 in 35,000 | |||
| 0.001 | |||||
| PLA microspheres | Gauthier (3 mLdilut.) | 1999–2002 | 15 in ∼1500 | 1.0 | 1.0 |
| Gauthier (5 mLdilut.) | 2002–2005 | 2 in ∼1500 | 0.13 | ||
| Bauer | 2000–2004 | 5 in 722 | 0.7% | ∼ 0.25 | |
| Vleggar | 2000–2003 | 3 in 2131 | 0.14 | ||
| Aventis Germany ** | 1999 – 2004 | ? in 150,000 | 0.2 | 0.2 | |
| HEMA particles | DeGoursac ** | 1998–2000 | 17 in > 800 | 2.1 | |
| Bergeret | 1998–2000 | 9 in 455 | 2.0 | ∼ 1.25 | |
| Harrer ** | 1998–2004 | 10 in 1630 | 0.6 | ||
| DermatechFrance ** | 1998–2005 | ? in 170,000 | 0 .225 | 0.22 | |
| Silicone oil | Greenberg | 1980–1990 | 1 in ∼1000 | 0.10 | |
| Orentreich | since 1985 | 1 in ∼5000 | 0.02 | 0.12 | |
| Fulton | 2002–2005 | 5 in 608 | 0.82 | ||
| Jones | 2003–2005 | 1 in 500 | 0.20 | ||
* Based on our interpretation of granulomas used in this presentation we classified ‘sterile abscess’ and ‘chronic inflammation’ as granulomas if they occur at least 2 months after injection at all sites at approximately the same time
Differentiation of nodules from granulomas
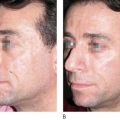
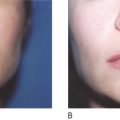
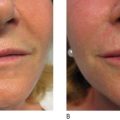
All injectables carry the danger of being over-injected, remodeled or dislocated when deposited into or close to a facial muscle. In a similar way that the muscle of a shell forms a pearl, constant muscle movement can form a nodule or ‘grain’ from an incorrectly deposited strand. This may be particularly obvious in the corners of the mouth ( Figure 12.3A ) and in the soft tissues of the lips ( Figure 12.3B ), where the microdroplet technique may eventually circumvent this ( ). The formation of these nodules can be blamed on inadequate implantation technique and must not be confused with true granulomas. By the same token, a sclerosing granuloma may mimic a nodule. Without biopsy, it may be impossible to prove and it is certainly not necessary to biopsy palpable, but not visible nodules.
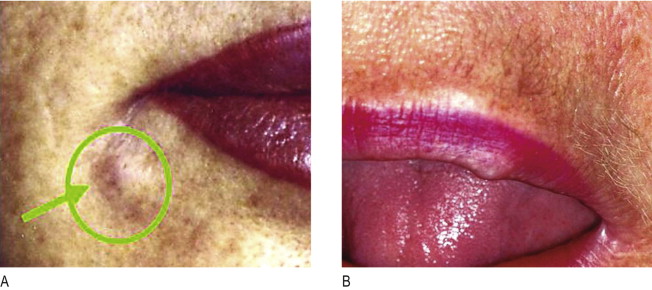
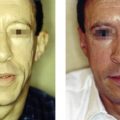
Nodules are isolated, single lumps in the implanted area ( Figures 12.3 ), which do not grow, and whose fibrous capsule confines them well from the surrounding tissue. Often white and harder than a genuine granuloma because they contain fewer cellular elements ( Figure 12.4A ), they are palpable or visually evident a few weeks post-injection. The histology of implant nodules reveals the appearance of a dense foreign material, macrophages and giant cells, which is a normal, deliberate foreign body ‘reaction ( Figure 12.4B ) similar to that described as foreign body granulomas ( , ). Giant cells, per se, are not the typical sign of granulomas but rather of a foreign material which is too big to be engulfed by macrophages. These fuse into giant cells to be more powerful ( Figure 12.5A ). Unfortunately, the pathological misdiagnosis of an intended normal foreign body reaction adds to the confusion for clinicians ( ).
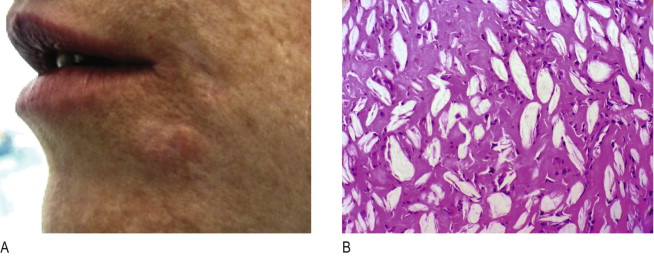
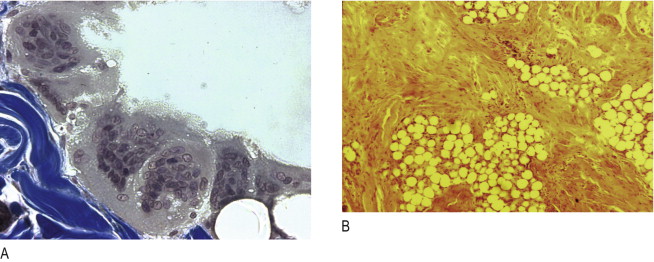
Another common misdiagnosis occurs when a dermal filler containing microspheres has been implanted too superficially into the dermis (instead of at the dermal–subdermal junction). In some cases, 2 or 3 months after implantation, a hyperpertrophic-type reaction of the skin may take place, which clinically and histologically resembles a hypertrophic scar or a ‘keloid’ with the typical colouring of the skin. The predominant components present in such cases are fibroblasts and broad collagen strands ( Figure 12.5B ) that are pushing the particles into clusters, not macrophages and giant cells as in true granulomas. These ‘hypertrophic scars’ react well to intralesional corticosteroid injections ( ).
The normal histology of implant material
Since foreign body granulomas caused by dermal fillers have not yet been introduced in modern pathology textbooks and are rarely mentioned in pathology literature ( ), most histopathologists will diagnose a normal and deliberate foreign body reaction to particulate material as a foreign body granuloma ( , , ). The confusion stems from the fact that particulate materials are implanted intentionally in order to stimulate a ‘foreign body reaction’, i.e. the ingrowth of cells and the encapsulation of each particle or microsphere with fibrous tissue, thus ensuring a softer and more pliable implant. It is not until something becomes symptomatic that the diagnosis of granuloma is entertained.
All injected substances cause an initial influx of mononuclear cells. In the case of a dermal filler containing microspheres, straight after implantation, macrophages are initially attached to the particles or microspheres, converting occasionally into giant cells (5–10 giant cells in a field at ×100 magnification is normal). If the particles are permanent and not constantly irritating, after 6–12 months, most giant cells will diminish in number and the histological picture will remain stable ( ).
In other resorbable implants such as Sculptra™, Dermalive®, or Radiesse®, the hyaluronic acid or methylcellulose carrier dissipates soon after injection and leaves the particles or microspheres packed with little space for tissue ingrowth ( ). The resorbable particles or microspheres are broken down enzymatically and are subsequently phagocytosed by macrophages and giant cells within 6–12 months after injection ( ).
The diagnosis of a true granuloma
Three different clinical and histological types of granulomas occur:
1. Cystic (or inflammatory, necrobiotic or palisaded) granulomas can develop superficially after intradermal biological gels such as collagen and hyaluronic acid injections ( Figure 12.2A ) and also after artificial gels like silicone or polyacrylamides ( Figure 12.6 ). They occur 2–12 months later or years after the injection of an artificial gel. Extreme inflammation, swelling and pain are the predominant clinical signs. Although implantation of bovine collagen is considered to be one of the least toxic and irritating biomaterials known ( ), its late complications have been described as ‘palisaded foreign body granuloma’ ( ) surrounded by a zone of neutrophils, lymphoid cells, macrophages, and a significant number of giant cells ( Figure 12.5A ), which are uncharacteristic of a bacterial abscess ( ). Necrobiotic granuloma has also been used to describe the collagen implant with collagen floating in a sea of neutrophils ( ).