Key Terms
Solar Lentigo
Lentigo senilis
Lentigo solaris
Simple Lentigo
Labial melanotic macules
Lentigo simplex
Melanonychia striata
Simple lentigo
Acquired Melanocytic Nevus
Acquired moles
Acquired nevi
Compound nevus
Intradermal nevus
Junctional nevus
Nevus Spilus
Speckled lentiginous nevus
Atypical (Dysplastic) Nevus
Atypical nevi
Clark nevi
Dysplastic nevi
Lentigo Maligna
Hutchinson freckle
Pigmented lesions include not only melanocytic neoplasms, such as nevi and melanoma, but also some pigmented keratinocytic processes, such as pigmented seborrheic keratoses, and some hamartomas, such as a Becker nevus. The evaluation of pigmented lesions and hence, this chapter, is especially important because a delayed or missed diagnosis of melanoma may lead to morbidity, or even mortality, in a patient.
Important History Questions
How long has this lesion been present?
This is an obvious but often overlooked inquiry. The patient’s response may range from useful to uncertain to flat-out misleading. Moreover, the development of a “new” lesion is not indicative of only melanoma but it should prompt further inquiry and a more detailed examination of the lesion.
Has the lesion changed?
Any change in a melanocytic lesion should also prompt a more detailed and careful physical examination.
Has the lesion bled?
Benign lesions can be traumatized, with resultant bleeding, but an affirmative response should raise the index of suspicion with regard to cancer.
Do you have a history of an atypical dysplastic nevus or melanoma?
Persons with atypical nevi and/or past melanoma are at increased risk for melanoma.
Is there a family history of atypical nevi or melanoma?
There is a familial tendency to develop atypical moles, which increases one’s personal risk of melanoma. Also, a first-degree relative with melanoma increases one’s own risk of developing melanoma.
Do you have a history of indoor tanning bed use?
Tanning bed use is associated with an increased risk for melanoma, especially in less common areas, like the buttocks.
Have you ever had sunburn that produced blisters, and, if so, when did you burn?
Persons with multiple blistering sunburns, particularly in youth, are more likely to develop melanoma.
Important Physical Findings
What is the patient’s skin type?
Persons with fair skin, blue eyes, and light-colored hair are more likely to develop melanoma. Persons with red hair have an elevated risk of melanoma, and melanoma in this population may be amelanotic.
How many moles does the patient have on his or her body?
A large number of nevi, whether atypical or dysplastic in appearance, or not, are at increased risk for melanoma. Most patients with an atypical/dysplastic nevus syndrome usually have more than 100 nevi.
Do any of the pigmented lesions on a patient’s body look different from the rest?
Sometimes called the ugly duckling sign , it is always useful to identify any pigmented lesion that looks remarkably different from the patient’s other pigmented lesions.
Do any of the pigmented lesions on a patient’s body violate the ABCDEs of melanoma?
These are: A, asymmetry, B, border irregularity, C , color variegation (multiple colors), D , diameter more than 6 mm (about the size of a pencil eraser), and E, evolutionary behavior (e.g., growing, bleeding, itching, burning). This does not mean that anything that violates a component of the ABCDEs is melanoma, but it does indicate that such a lesion must be carefully evaluated, and possibly biopsied, to exclude melanoma.
Solar Lentigo
ICD10 code L81.4
BENIGN NEOPLASIA
Pathogenesis
A solar lentigo, also referred to as lentigo solaris and lentigo senilis , results from hyperpigmentation of keratinocytes, with a slightly increased number of singular melanocytes. Lentigines are caused by increased cumulative ultraviolet (UV) light exposure. This latter characteristic explains the photodistribution of lentigines and the appearance of lentigines in later life. Although the term liver spot is occasionally used by laypersons, it is a term that should be discouraged because lentigines have nothing to do with liver disease.
Clinical Features
- •
Most solar lentigines occur in persons with fair skin.
- •
Solar lentigines occur on photoexposed skin, such as the head and neck, upper anterior chest, forearms, and dorsal hands.
- •
Solar lentigines are usually multiple and are associated with dermatoheliosis (sun-damaged skin).
- •
Solar lentigines are highly variable in size, ranging from 1 mm to larger than 1 cm.
- •
Solar lentigines exist as a round or oval macule or patch, which is tan to dark brown. Rare lesions may be black ( Figs. 31.1–31.3 ).
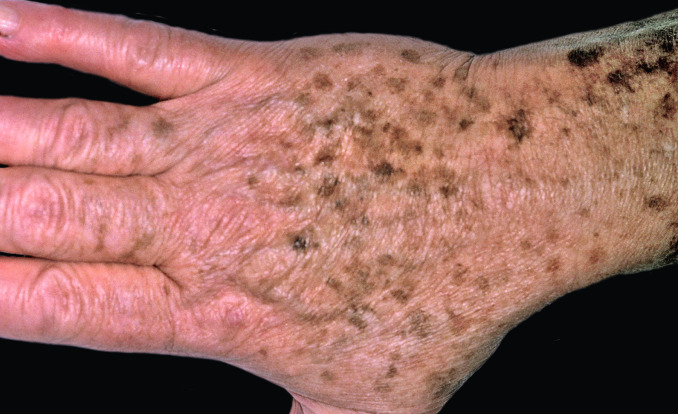
Fig. 31.1
Sun-damaged atrophic skin on the back of a patient’s hand with numerous solar lentigines that vary in color from tan to brown to almost black. The area of more proximal discoloration represents solar purpura.
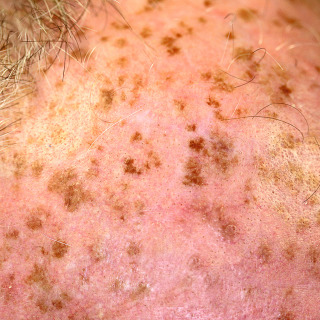
Fig. 31.2
Patient with sun-damaged atrophic skin on the scalp, with numerous solar lentigines that vary in color from tan to brown. The area of yellowish discoloration represents solar elastosis, and the inflamed scaly lesion is an actinic keratosis.
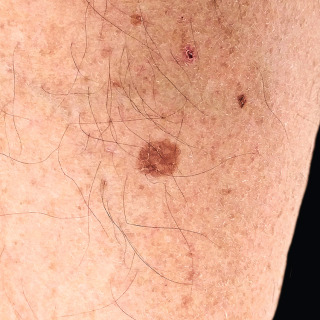
Fig. 31.3
Patient with mildly sun-damaged skin on the leg, with one brown, slightly scaly solar lentigo and a smaller solar lentigo.
- •
Solar lentigines are usually symmetric, but some irregularities may be present on occasion.
Diagnosis
- •
The occurrence of multiple, uniform, tan to brown macules and small patches on the sun-exposed skin of a fair-skinned person with sun damage is usually diagnostic.
- •
The differential diagnosis includes melanoma in situ (lentigo maligna type), which also occurs in older persons and on sun-damaged skin. In general, melanoma in situ (lentigo maligna type) is larger, with greater irregularity and with more variegated color.
- •
In problematic cases, a biopsy can be done to exclude melanoma in situ (lentigo maligna type).
Treatment
- •
No treatment is required for lentigines.
- •
Broad-spectrum sunscreen and other sun protective measures will lessen melanin production and prevent further sun damage.
- •
If ablation is desired, one can use liquid nitrogen cryotherapy, usually with only about a 5-second freeze to avoid overtreatment. Destructive therapy of pigmented lesions should be avoided unless the assessment of a benign condition is rendered with the utmost confidence.
- •
Lasers targeting melanin can be used in select cases, but this is generally done by a specialist.
- •
Chemical peels and topical bleaching formulations, such as 2% to 6% hydroquinone or 2% mequinol and 0.01% tretinoin (Solage) can be used, but, again, the assessment of benign lentigo must be made with the utmost confidence. Often bleaching from topical agents is incomplete.
Clinical Course
Solar lentigines, when confidently assessed, are benign and require no treatment. Lentigines can be difficult to differentiate from melanoma in situ (lentigo maligna type). Lentigines usually persist and may even increase in number over time with continued sun exposure.
Simple Lentigo
ICD10 code L81.4
BENIGN NEOPLASIA
- •
Carney complex: blue nevi, atrial myxomas, and endocrinopathies
- •
Centrofacial lentiginosis syndrome: lentigines, spina bifida, mental and learning disorders
- •
Leopard syndrome: electrocardiographic abnormalities, ocular hypertelorism, pulmonary stenosis, abnormalities of the genitalia, retardation of growth, deafness
- •
Peutz-Jeghers syndrome: intestinal polyposis
Pathogenesis
In contrast to a solar lentigo, a simple lentigo, or lentigo simplex, is unrelated to sun exposure. Simple lentigines are genetically predetermined and may be seen in some syndromes (see box). Simple lentigines, in contrast to solar lentigines, may occur on mucosal surfaces, such as the lips and genitalia, and are caused by increased melanocytes at the dermoepidermal junction, with increased production of melanin. There is often elongation of epidermal rete ridges as well. Simple lentigines lack genetic mutations related to UV light exposure.
Clinical Features
- •
Simple lentigines can arise at any age, but most cases occur in children and young adults.
- •
Simple lentigines can be solitary ( Fig. 31.4 ) or multiple. When lesions are numerous, consider evaluation for a syndrome ( Figs. 31.5–31.7 ).
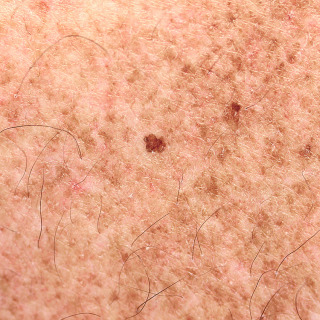
Fig. 31.4
Patient with slightly irregular simplex lentigo on sun-damaged skin. Simple lentigines can arise on sun-exposed skin or non–sun-exposed skin. This one was removed for histologic diagnosis.
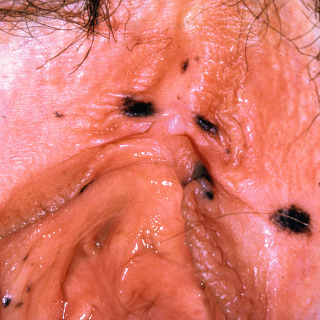
Fig. 31.5
Multiple genital lentigines in a woman with Carney complex.
(From the Fitzsimons Army Medical Center Collection, Aurora, CO.)
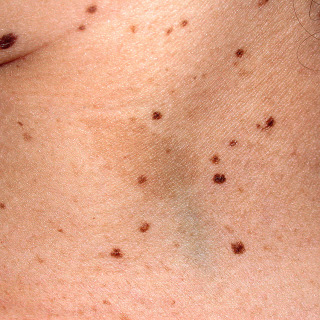
Fig. 31.6
Patient with centrofacial lentiginosis syndrome. Also present is a café au lait macule.
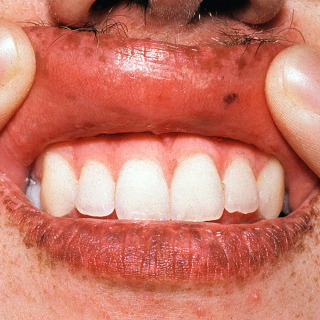
Fig. 31.7
Multiple lentigines on the lip and oral mucosa in a patient with Peutz-Jeghers syndrome.
(From the Fitzsimons Army Medical Center Collection, Aurora, CO.)
- •
Simple lentigines can occur on any site, but the palms, soles, genitalia, and lips are common sites.
- •
Simple lentigines are usually uniform, round to oval macules, brown to black in color, about 1 to 4 mm in size. Rarely, larger lentigines exist.
- •
Oral mucosal lentigines are often called labial melanotic macules . Genital lentigines may occur as well. In the nail unit, a pigmented streak caused by a lentigo is referred to as melanonychia striata .
Diagnosis
- •
The homogeneous and banal character of simple lentigines is usually diagnostic.
- •
The differential diagnosis of simple lentigines includes junctional nevi and solar lentigines; the latter occurs on sun-damaged skin and in older persons.
- •
The diagnosis of a simple lentigo, particularly as distinguished from a junctional nevus or malignant melanocytic process, can be established by biopsy.
Treatment
- •
Simple lentigines, when confidently assessed, require no treatment and are benign entities.
- •
As with all pigmented proliferations, if the lesion continues to enlarge, darken, or bleed, the patient should return for re-evaluation considering the changing nature of the process.
- •
If removal is desired, a shave, punch, or excision biopsy is often the treatment of choice.
Clinical Course
Simple lentigines, like all melanocytic processes, could, in theory, progress to malignant melanoma, but the risk is extremely small. Simple lentigines are not considered premalignant.
Acquired Melanocytic Nevus
ICD10 code D22 (site dependent)
BENIGN NEOPLASIA
Pathogenesis
Acquired melanocytic nevi, also known as acquired nevi , or acquired “ moles ,” are the most common of benign melanocytic neoplasms. Acquired nevi develop in early childhood and increase in number until adulthood, only to then regress, and decrease in number, in later adulthood. Several factors, including a family history of numerous nevi, light skin, light hair and eye color, and greater sun exposure in youth, are associated with increased nevi. In a longitudinal study of children in Colorado, at 8 years of age, boys had about 30 melanocytic nevi, whereas girls had about 25 melanocytic nevi. Pigmented melanocytic nevi may exhibit nests of cells only at the dermoepidermal junction (junctional nevus), only in the dermis (intradermal nevus), or in both locations (compound nevus). Intradermal nevi are not usually pigmented.
Clinical Features
- •
Acquired melanocytic nevi appear first in early childhood and increase in number until adulthood.
- •
Most junctional or compound nevi occur on skin that is intermittently or chronically sun-exposed.
- •
Junctional nevi are usually tan to black in color, 1 to 6 mm in size, and with a round or oval shape and sharp circumscription.
- •
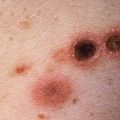
Compound nevi are usually raised papules, tan to dark brown in color, with sharp circumscription and regular margins ( Figs. 31.8–31.10 ). Their size is variable, with most compound nevi being between 2 and 8 mm in size, but larger compound nevi are not uncommon (see Fig. 31.10 ).
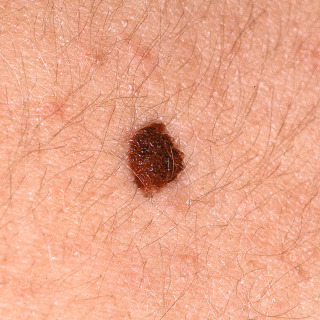
Fig. 31.8
Patient with a dark brown, relatively symmetric compound nevus.
(From the Joanna Burch Collection, Aurora, CO.)
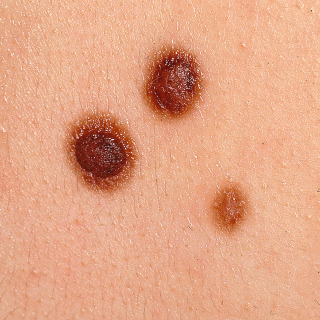
Fig. 31.9
Patient with two compound nevi with a fried egg appearance and a flat macular junctional nevus.
(From the Joanna Burch Collection, Aurora, CO.)
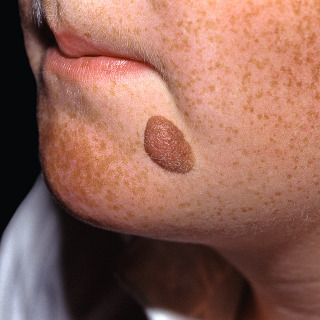
Fig. 31.10
Unusually large acquired, light brown, symmetric compound nevus. The patient also has numerous freckles.
(From the Joanna Burch Collection, Aurora, CO.)
- •
Acral nevi tend to be junctional in nature and are often heavily pigmented ( Fig. 31.11 ).
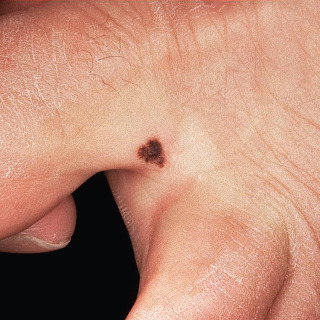
Fig. 31.11
Acquired acral junctional nevus in an adult, with an irregular outline. This lesion was removed for histologic examination.
(From the Fitzsimons Army Medical Center Collection, Aurora, CO.)
- •
Thickened dark hairs may be prominent in larger compound nevi.
- •
Melanocytic nevi may darken during pregnancy in response to elevated estrogen levels.
Diagnosis
- •
Most benign melanocytic nevi may be diagnosed based upon the clinical appearance, but sometimes it can be difficult to distinguish benign nevi from pigmented seborrheic keratoses or melanoma. When patients have multiple nevi, most tend to be roughly similar in appearance and size, and outliers should be treated with greater suspicion (the so-called “ugly duckling sign”).
- •
If there is concern regarding a pigmented lesion, a deep shave, punch, or excisional biopsy should be performed. A punch biopsy or excision that removes the pigmented lesion completely allows the pathologist or dermatopathologist to examine the lesion in its entirety and prevents recurrences.
Treatment
- •
No treatment is required for lesions that may confidently be assessed as benign nevi.
- •
New or changing lesions, particularly those occurring after 40 years of age, unusually large lesions, irregular or inflamed lesions, and/or lesions substantially different from others on the body should be biopsied.
Prognosis
It is thought that melanocytic nevi regress or involute in the elderly. One study reported that by age 70 patients had an average of two melanocytic nevi on their bodies, although one author (WAH), who maintains a textbook on geriatric dermatology, and routinely sees older patients, believes this is exaggerated. It has been estimated that the rate of development of melanoma in a wholly benign nevus may be as low as 1:100,000/year.
Congenital Melanocytic Nevus
ICD10 code D22 (site dependent)
BENIGN NEOPLASIA
Pathogenesis
The pathogenesis of congenital melanocytic nevi is poorly understood, but studies have suggested congenital melanocytic nevi behave differently from acquired melanocytic nevi. For example, acquired nevi are monoclonal populations of nevomelanocytes, whereas congenital melanocytic nevi are comprised of multiple clones. It has been estimated that about 1% to 5% of the population has a congenital nevus.
Clinical Features
- •
Congenital nevi are present at birth.
- •
Nevi that develop in the first 2 years of life may be called congenital nevus–like melanocytic nevi.
- •
A new classification system was proposed in 2013, but the former classification system is still the most widely used by most dermatologists:
- –
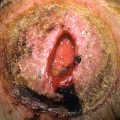
Small—less than 1.5 cm ( Fig. 31.12 ), with a risk of malignant degeneration equal to acquired nevi.
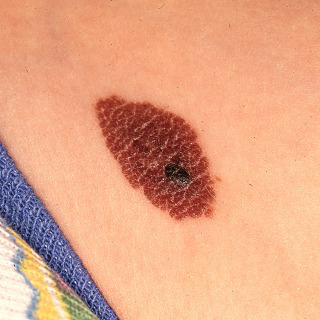
Fig. 31.12
Small oval congenital nevus on the upper thigh of an infant. The smaller, darker papule should be carefully monitored for change.
- –
Medium—less than 1.5 to 19.9 cm ( Fig. 31.13 ), with an elevated but imprecisely known risk of malignant degeneration.
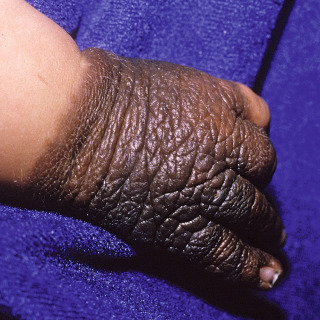
Fig. 31.13
Patient with a medium-sized congenital nevus of the hand.
(From the Fitzsimons Army Medical Center Collection, Aurora, CO.)
- –
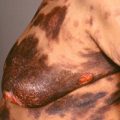
Large (giant)—larger than 19.9 cm ( Figs. 31.14 and 31.15 ), with an estimated rate of malignant degeneration of about 5% to 10%/lifetime.
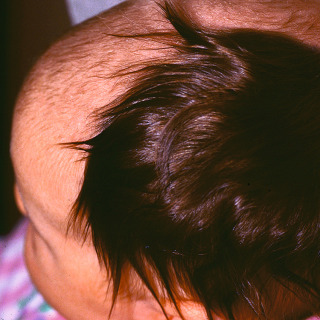
Fig. 31.14
Patient with a giant congenital nevus of the scalp, with enlarged dark hairs.
(From the Joanna Burch Collection, Aurora, CO.)
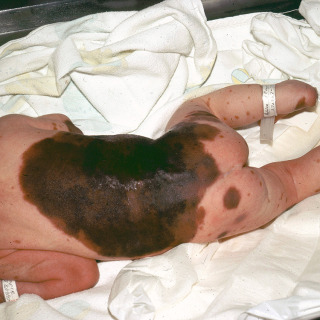
Fig. 31.15
Patient with a giant congenital nevus, with numerous smaller satellite congenital nevi.
(From the William Weston Collection, Aurora, CO.)
- –
- •
Large congenital nevi may demonstrate one or more smaller satellite lesions (see Fig. 31.15 ).
- •
Congenital nevi with satellite lesions, or those overlying the spine, are more likely to be associated with neurocutaneous melanosis, with hydrocephalus in two-thirds of patients, and with possible seizures.
Diagnosis
- •
The diagnosis of a congenital nevus is usually established on clinical and historical grounds.
- •
In some cases, a punch or incisional biopsy can be done for histologic examination, particularly if there is concern for malignant degeneration in a particular area.
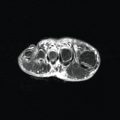
- •
Some authorities recommend that brain or spinal magnetic resonance imaging (MRI) be performed in the setting of large congenital nevi because of the risk of central nervous system (CNS) involvement (neurocutaneous melanosis), but other authorities reasonably question the value of such imaging because there is no adequate treatment for neurocutaneous melanosis.
Treatment
- •
If any congenital nevus demonstrates changes in size, color, or shape, or if there is a new nodule, ulceration, or features of regression, the lesion must be examined by an expert. It is likely that a biopsy will be performed, which should be examined by a dermatopathologist with expertise in this area.
- •
There is no standardized management for congenital melanocytic nevi that are stable.
- •
For small and medium-sized lesions, it is often reasonable to adopt a wait and watch approach, instructing patients and family members to monitor lesions, in cooperation with a health care provider.
- •
Large or giant congenital nevi are problematic because of the definitive increased risk of malignant degeneration, yet prophylactic excision may be surgically problematic. Ultimately, the risks of general anesthesia in any surgical intervention(s) must be balanced against the risk for melanoma arising in the lesion.
Clinical Course
The risk of malignant melanoma arising in a congenital melanocytic nevus is difficult to calculate with certitude for any individual lesion. Older classification schemes fostered this imprecision due to wide ranges in category sizes, but in general, the larger the lesion, the higher the risk of malignant degeneration. The lifetime risk for malignant melanoma developing in a giant congenital nevus may be as high as 5% to 10%. The risk for medium-sized congenital nevi, and even small congenital nevi, is clearly lower, but studies have reported conflicting data.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree