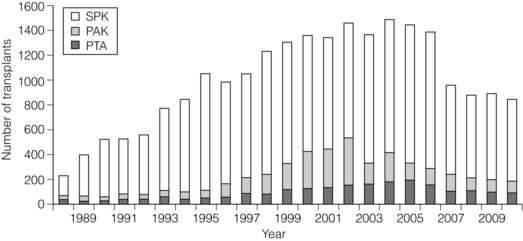
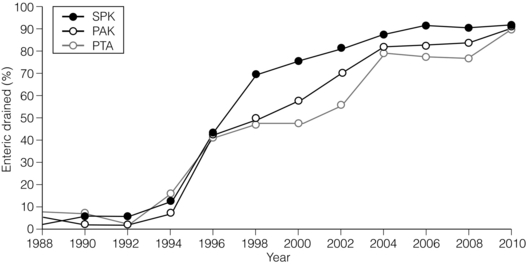
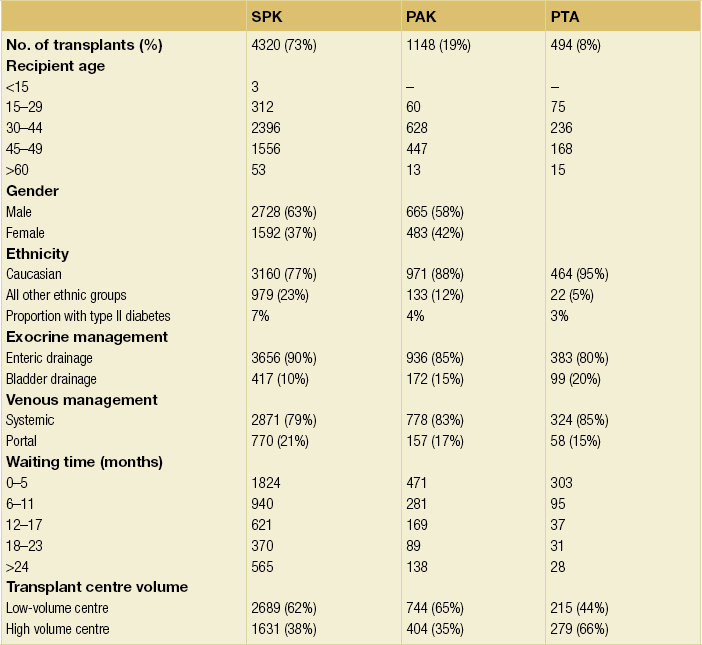
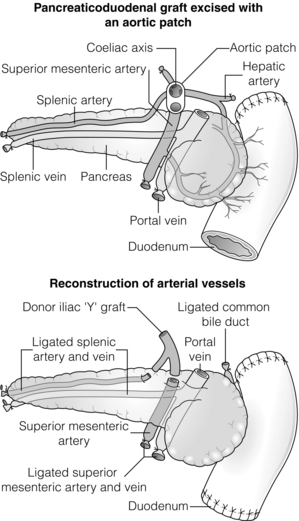
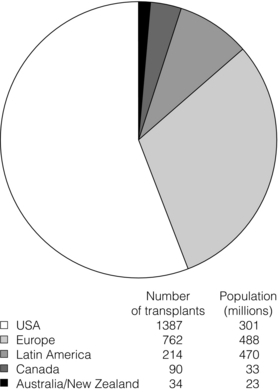
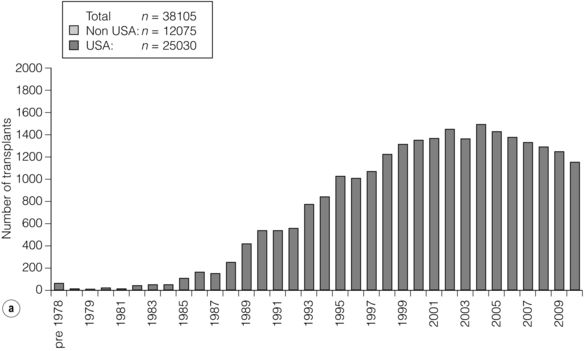
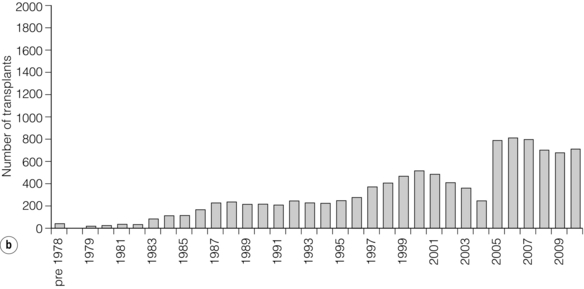
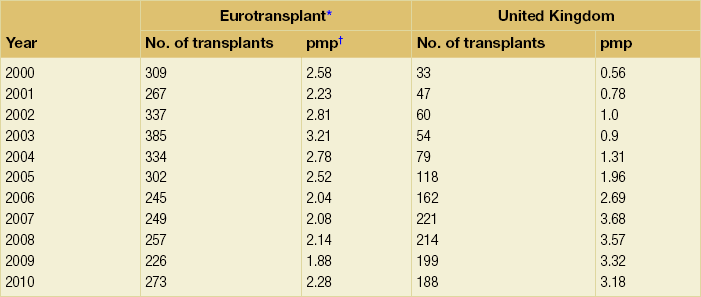
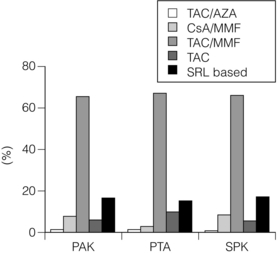
9 The first pancreas transplant was performed in the University of Minnesota in 1966.1 Early experience with pancreatic transplantation was disappointing. This remained so for many years. Difficulties were related to the management of the exocrine secretions and septic complications, a high incidence of thrombosis, acute rejection and pancreatitis. For the first half of its 45-year history, less than 1200 pancreas transplants were performed worldwide. Even after the introduction of ciclosporin, in 1983, 1-year patient and graft survival rates were only 75% and 37%, respectively. Understandably, in the 1970s and 1980s enthusiasm for pancreas transplantation was scarce; the predominant sentiment was scepticism. Pancreas transplantation for type II diabetes Pancreas transplantation aims to provide patients with type I diabetes with an alternative source of insulin. In 1998, Sasaki et al. reported a small number of patients with insulin-requiring type II diabetes who had received pancreas transplants with short-term success.2 This and a few other single-centre retrospective reports focused on small numbers of patients thought to have type I diabetes at the time of transplant, which were subsequently classified as type II. Whilst short-term outcomes after pancreas transplantation for type II diabetics appeared similar to those with type I diabetes, concern existed about the effect of older age of onset, metabolic syndrome, insulin resistance, higher prevalence of obesity and cardiovascular comorbidity in such patients. It also became clear that no clinical or biochemical test exists that can reliably distinguish type I from type II diabetes mellitus. Neither fasting nor stimulated serum C peptide levels predict insulin production, especially in the presence of renal failure and impaired excretion.3 Type II diabetes can present at a young age with signs of autoimmunity whereas some with type I diabetes can present later in life with no evidence of an autoimmune disorder. There are in fact reasonable grounds to question the validity of the view that type I and type II diabetes are two distinct disorders with different aetiology and pathogenesis. The ‘accelerator hypothesis’ considers both types of diabetes to be the same disease of insulin resistance where a specific genetic inheritance and some environmental and behavioural factors determine the age of onset.4 The International Pancreas Transplant Registry (IPTR) began recording data on type II diabetes in the mid-1990s. Until 5 years ago, there had been a slow increase in the proportion of simultaneous pancreas–kidney (SPK) transplant recipients reported as having type II diabetes, reaching 7%. This proportion has remained stable over the last 5 years.5,6 Most registry analyses confirmed that type II diabetics do as well as those with type I following pancreas transplantation. The pancreas transplant database of all US transplants performed between 1967 and 2011 records 72 living-donor transplants out of approximately 25 000 pancreas transplants (0.3%).6 Only three centres in the USA have performed this procedure, more than 80% of reported cases coming from the University of Minnesota. A recent review article7 by the authors of the largest series quotes the worldwide experience as approximately 160 cases, with only two centres outside the USA contributing several cases each. One-year pancreas graft survival after living-donor pancreas transplantation in the Minneapolis series was inferior to graft survival after deceased donor transplantation. Long-term outcome was comparable. The experience regarding perioperative morbidity and the long-term risks for the donor is inadequate to allow comment. It is of concern that only 67 of the first 115 live pancreas donors were traced to complete a follow-up survey. The survey revealed that three of these donors needed to start insulin some time after donation and 10 had elevated HbA1c levels. Pancreas transplantation is performed in three distinct clinical settings, as presented below. There is little debate that diabetic patients with renal failure should be offered kidney transplantation and there is good evidence that their prognosis is poor on dialysis.8 Such patients will already be obligated to immunosuppression on account of kidney transplantation. Combined kidney and pancreas transplantation offers these patients the opportunity to become insulin independent as well as dialysis independent. The risks and potential benefits of SPK transplantation compared with kidney transplantation alone for diabetic patients are summarised in Box 9.1. The evidence for the risks and benefits alluded to in Box 9.1 is reviewed in the final part of the chapter. Experience has taught us the crucial importance of recipient selection for success in pancreas transplantation. Cardiovascular comorbidity is the most important factor leading to patient death in the early postoperative period after transplantation in diabetic patients. Patient selection needs to include a comprehensive medical evaluation, ideally performed by a multidisciplinary team within each transplant unit. Asymptomatic ischaemic heart disease is not uncommon in diabetics. The cardiac assessment should include as a minimum a 12-lead electrocardiogram (ECG), echocardiography, exercise tolerance test and a non-invasive test of myocardial perfusion (a radioisotope scan or dobutamine stress echocardiography). There is insufficient evidence to comment on the value of routine angiography.9 Any patient with an abnormality detected in non-invasive tests or those with symptoms of ischaemic heart disease should be investigated further, including angiography. Any correctable coronary artery disease should be dealt with prior to transplantation.10 Two per cent of SPK transplants between 2006 and 2010 were performed in recipients aged 60 or older. Strong evidence to differentiate between contraindications and relative contraindications does not exist (Box 9.2). Neither blindness nor previous amputation are regarded as contraindications. Hepatitis B, hepatitis C or human immunodeficiency virus (HIV) infection in the potential recipient are not contraindications to pancreas transplantation either. Box 9.2 is intended as a guide only. No attempt has been made to separate absolute and relative contraindications. The use of imprecise definitions such as ‘significant’, ‘severe’ or ‘recent’ is also intentional. Appropriate patient selection requires a balanced assessment by experienced clinicians of the risk factors versus potential benefits. Historically, the large majority of pancreas transplants performed have been SPKs. This was largely because of the relatively poor outcomes following solitary pancreas transplantation. Improved success of pancreas transplantation in the last 15 years12,13 has encouraged many transplant units to offer pancreas transplantation for diabetic patients who have previously undergone successful kidney transplantation. Until the end of 2000, PAK transplants constituted 11% of all pancreas transplants performed in the USA and 5% of pancreas transplants performed outside the USA. Thereafter a rapid increase in PAK transplant rate was observed, reaching a peak of 400 (around a third of all pancreas transplants) in the USA in 2004.6,11 There has since been a sharp decline in PAK numbers in the USA. More detailed analysis of activity and outcome in different forms of pancreas transplantation is given in the next section. Contraindications to PAK transplantation are the same as those for SPK (Box 9.2). Within the confines of these contraindications, all diabetics who have previously undergone successful kidney transplantation are potentially suitable candidates for PAK. In practice the procedure is most useful for those diabetics who have a potential living donor for kidney transplantation. Since PAK outcomes are now approaching those of SPK, an elective and early living-donor kidney transplantation has obvious benefits to the potential recipient and has additional benefits to the overall pool of patients awaiting transplantation by releasing another deceased donor kidney. Clearly some time should elapse after kidney transplantation to allow for recovery from surgery and stabilisation of allograft function and immunosuppression. The optimal timing has not been determined. A study comparing early (within the first 4 months) with late (more than 4 months after kidney transplantation) PAK did not reveal any significant difference in the incidence of morbidity or outcome.14 More recent data suggest that longer intervals (greater than 3 years) between kidney transplantation and subsequent PAK transplantation can be associated with increased risk to the kidney graft.15 An important consideration, in particular for patients who are being assessed some considerable time after kidney transplantation, is the adequacy of kidney function. The level of kidney function at the time of PAK transplantation, independent of the time interval between the two transplants, is a factor associated with mortality risk.16 Criteria based on strong evidence do not exist but a creatinine clearance of greater than 40 mL/min is a useful guide as a minimum requirement.17 For recipients of kidney transplants who are not receiving calcineurin inhibitors, a higher creatinine clearance of not less than 55 mL/min is recommended. A renal allograft biopsy prior to PAK is useful for documentation of the baseline renal reserve and for the monitoring of the progression of allograft nephropathy (diabetic or otherwise). The discussion above relating to SPK and PAK highlights the difficult choice facing patients. The experience of the local pancreas transplant centre, long waiting times for SPK transplantation and individual risk factors (age, obesity, race, etc.) that may adversely affect pancreas transplant outcome may favour early live-donor kidney transplantation followed by PAK. On the other hand, most fit patients in experienced centres without unduly long waiting times are likely to fare better with SPK.15 Universally agreed criteria that constitute indications for PTA are more difficult to find compared with those for SPK or PAK. The risk of transplantation for non-uraemic diabetics needs to take account of not only perioperative morbidity but also long-term risks associated with immunosuppression. At present the majority of patients developing type I diabetes will be better served by insulin injections at the onset of disease. Clear indications for PTA are life-threatening complications of diabetes in patients managed with insulin, namely intractable hypoglycaemic unawareness and cardiac autonomic neuropathy. In these two subpopulations of diabetic patients pancreas transplantation is truly life saving. Other indications for PTA are more controversial. The presence of two or more diabetic complications has been advocated as an indication.18 In the absence of conclusive data on some of the diabetic complications, as reviewed in the final section of this chapter, it is difficult to defend the logic behind the assertion that two or more complications constitute an indication for PTA. Disabling and intractable symptoms of diabetic neuropathy or early nephropathy with preserved renal function may be considered as indications. In diabetics with overt nephropathy and impaired renal function, the nephrotoxicity of the immunosuppressive drugs will need to be considered. Available evidence does not permit precise guidelines but patients with creatinine clearance less than 40 mL/min may be better served by SPK transplantation. At the beginning of 2011 the total number of pancreas transplants performed worldwide was close to 40 000.6 Nearly two-thirds of these have been performed in the USA. The International Pancreas Transplantation Registry (IPTR) shares US transplant data with the United Network for Organ Sharing (UNOS). Reporting of data to the UNOS is compulsory, hence IPTR data regarding US pancreas transplantation activity are accurate and complete. Non-US pancreas transplants are reported to the IPTR on a voluntary basis. Some national organisations, such as Eurotransplant, share data with the international registry. The IPTR estimates that around 90% of non-US transplants are reported. Worldwide pancreas transplantation activity reported in a Council of Europe document suggests19 that a much greater proportion of non-US transplants are missing from the IPTR database (Fig. 9.1). Despite this probable under-representation of non-US transplants, there seems to be a genuine difference in the utilisation of pancreas transplantation between the USA and the remainder of the world. Figure 9.2 shows worldwide pancreas transplantation activity as recorded by the IPTR at the beginning of 2011.6 Figure 9.1 Pancreas transplants performed worldwide in 2006. Data from International Figures on Organ Donation and Transplantation 2006. Transplant Newsletter 12(1), September 2007. Council of Europe. Figure 9.2 Pancreas transplants reported to the International Pancreas Transplant Registry (1 January 2011). (a) USA. (b) Non-USA. In the USA, SPK transplantation activity reached a peak of 972 transplants in 1998. Thereafter there has been a continuous slow decline in SPK activity, with just over 800 SPK transplants recorded in 2010. Solitary pancreas transplantation activity (PAK + PTA) continued to increase until 2004, reaching a peak of 520 transplants in 2004 (Fig. 9.3). Since then there has been a decline. The decline in the number of solitary pancreas transplants, in particular PAK transplants, has been sharper than the reduction in SPK numbers. In 2010 less than half as many PAK transplants were performed in the USA compared with 2004. The total numbers of pancreas transplants performed in the UK and in the Eurotransplant zone are shown in Table 9.1 for comparison.20,21 In Europe, within the Eurotransplant zone, pancreas transplantation activity has declined slowly since the peak of 2003. In the UK pancreas transplantation activity reached a peak in 2007 and some decline has occurred since. Pancreas transplant rate (the number of transplants per million population per annum) in the UK exceeded that of the Eurotransplant area in 2006 and has remained well above the Eurotransplant rate since then. Table 9.1 Pancreas transplantation activity in the Eurotransplant area and in the United Kingdom *Eurotransplant zone includes Austria, Belgium, Croatia, Germany, Luxembourg, the Netherlands and Slovenia. The age profile of pancreas transplant recipients has changed markedly over the last two decades. In the USA between 1987 and 1992, 9% of pancreas transplant recipients were older than 45. This proportion had risen to 34% in 2000–2004 and further to 41% in 2006–2010.6,11 Portal venous drainage gained in popularity in the mid-1990s. In the USA between 1996 and 2002, 1091 of the 4394 (25%) enterically drained primary SPK cases have been performed with the portal venous drainage technique.6 There has since been a decline in the utilisation of portal venous drainage, with recorded rates of 18% in SPK and PAK transplants and 10% in PTA transplants in 2010.6 Other demographic data for patients transplanted in the USA between 2004 and 2009 are summarised in Table 9.2. Criteria for eligibility for pancreas donors Contraindications to organ donation are clinical HIV infection, diagnosis of Creutzfeldt–Jakob disease (CJD), history of malignancy (except non-melanoma skin cancer and certain primary central nervous system tumours) and active systemic sepsis. Additional specific contraindications to pancreas donation are the presence of diabetes mellitus or gross pancreatic disease in the donor (including trauma, acute or chronic pancreatitis, excessive fatty infiltration). Neither hyperglycaemia nor hyperamylasaemia should preclude pancreas donation providing the pancreas appears normal on inspection. Increasing donor age, cerebrovascular/cardiovascular cause of death and donor obesity are associated with poorer pancreas transplant outcomes.22–24 A precise upper age limit for pancreas donors is difficult to define because of many confounding variables, the retrospective nature of the studies and small sample sizes of individual reports. IPTR analyses use 45 as the age cut-off distinguishing ‘young’ and ‘old’ donors. Historically no more than 3% of US pancreas donors were older than 49 and this has remained largely unchanged.6,11 In practice an upper age limit of around 55 is used by most transplant units worldwide. The general consensus is that the ideal pancreas donor will be younger than 45. It is similarly difficult to quote an acceptable weight limit for donors. Body mass index (BMI) > 30 should be regarded as a relative contraindication. Pancreas grafts from paediatric donors (age > 4 years) and selected non-heart-beating donors can be used with excellent results.25,26 The influence of donor related factors on pancreas transplant outcome is discussed on p. 168. University of Wisconsin (UW) solution was first developed as a pancreatic preservation solution27 and remains the benchmark for pancreas preservation. Other preservation solutions have been used but experience with these is more limited.28 Recent experience with histidine–tryptophan–ketoglutarate (HTK) solution and Celsior solution suggests that both these solutions perform equally well as UW solution with short (< 12 h) ischaemia times. UW solution may perform better in longer cold ischaemia times exceeding 12 hours (for further details see Chapter 6).29 The cold ischaemia tolerance of the pancreas is somewhere between that of the liver and the kidney. In pancreas allografts perfused with UW solution, 20 hours was thought to be the limit for successful preservation, beyond which a time-dependent deterioration in outcome occurred.30 Earlier data failed to demonstrate a clear benefit from a preservation time cut-off below 20 hours. Most surgeons intuitively aim for shorter preservation times. The mean (± SE) preservation time for the 2000–2004 cohort of pancreas transplants in the USA was 13.3 (± 5.9) hours for SPK, 15.3 (± 5.5) hours for PAK and 16.2 (± 5.8) hours for the PTA group.31 More recent data suggest that ischaemia time is of greater importance in recipients of suboptimal grafts and in such cases ischaemia times over 12 hours are likely to be associated with poorer outcomes. Registry data reveal that pancreas transplant surgeons have taken this message on board, resulting in a trend towards shorter preservation times. The median cold ischaemia time for all US pancreas transplants has been under 12 hours since 2006.32,33 The pressure gradient between mean arterial pressure and portal venous pressure that maintains blood flow through the pancreas can be significantly diminished during the perfusion of the abdominal organs in retrieval operations. Particular attention is required to maintain an adequate gradient if a cannula for perfusion is placed in the portal venous system as well as the aorta. Many transplant units perfuse abdominal organs with an aortic cannula only. Some evidence supports the view that additional portal perfusion is unnecessary.34 For the interests of the pancreatic allograft, aortic perfusion alone is the most ‘physiological’ state that allows satisfactory perfusion and adequate drainage of the effluent. Povidone–iodine during cold storage may be toxic to duodenal mucosa.35 If povidone–iodine is used for flushing the allograft duodenal segment during organ retrieval, further flushing of the duodenal segment with preservation solution on the back table should be considered. Donor duodenal contents should be submitted for bacterial and fungal culture. The results may be important in guiding the management of infection in pancreas transplant recipients.36 • Careful and minimal handling of the pancreas during retrieval is important. Removal of the spleen and the pancreatico-duodenal graft en bloc with the liver is the quickest and safest method for both organs. The organs are then easily and quickly separated on the back table at the retrieval centre. • Further back-table preparation of the pancreas, which takes place in the recipient centre, is a crucial part of the procedure and takes a minimum of 2 hours. The short stumps of the gastroduodenal artery (GDA) and the splenic artery should be marked with fine polypropylene sutures at the time of retrieval. Demonstration of good collateral circulation within the pancreatico-duodenal arcade (between the superior mesenteric artery (SMA) and the GDA) by flushing the arteries individually at the back table is reassuring. An iliac artery Y graft of donor origin anastomosed to the SMA and the splenic artery is the most common method of reconstruction for the graft arterial inflow (Fig. 9.5). Meticulous dissection and ligation of the lymphatic tissue and small vessels around the pancreas is important to prevent haemorrhage upon reperfusion of the graft in the recipient. Particular attention should be paid to secure the duodenal segment staple lines by inversion with further sutures. • segmental pancreas transplantation using only the tail and the body of the pancreas; • pancreatic duct occlusion or ligation; • free drainage of exocrine secretions into the peritoneal cavity; • direct anastomosis of the transected pancreatic head into the bladder; Currently the choices available to the surgeon are related to the management of the exocrine secretions and the management of the venous drainage, as discussed below. Any part of the recipient’s small bowel can be used for anastomosis with the allograft duodenum. No data exist to demonstrate the superiority of one particular site over others. Roux-en-Y loops, which were commonly used, are becoming rare and a simple side-to-side entero-enterostomy is preferred.31 Drainage of the venous outflow from pancreas grafts into the portal circulation was first described by Calne in 1984.37 This complex surgical technique using a segmental graft and gastric exocrine diversion in a paratopic position has never gained popularity. Drainage of the venous outflow into the systemic circulation at the level of the lower inferior vena cava has been the norm in pancreatic transplantation. Since the mid-1990s there has been a resurgence of interest in portal venous (PV) drainage. The technique described by Shokouh-Amiri et al.38 involves placement of the graft slightly more cranially in the abdominal cavity, with the utilisation of the superior mesenteric vein (SMV) for the venous drainage. Several studies, including prospective randomised comparisons, have shown that this offers at least equivalent outcome to that of systemic venous (SV) drainage, with no compromise in safety.39–41 The impetus for PV drainage was to achieve a more physiological insulin delivery. A theoretical benefit was considered to be avoidance of hyperinsulinaemia, which has been linked with atherogenesis.42 Systemic drainage does not always cause hyperinsulinaemia. Nor may it be the only factor, since hyperinsulinaemia occurs in non-diabetic recipients of kidney transplants receiving steroids.43,44 None of the studies of metabolic function after PV drainage have shown a clear benefit in terms of glucose metabolism, lipid profiles or atherogenesis. There is some evidence suggestive of an immunological advantage to PV drainage in the form of a reduction in the incidence of acute rejection. The mode of antigen delivery is known to modulate the immune response, which has been proposed as the mechanism responsible for the observed reduction in acute rejection rates in portal venous drained grafts.39–41 Historically there is ample evidence that the incidence of acute rejection is higher after pancreas transplantation compared with kidney transplantation.18,30 The reasons for this difference are not clear. Nevertheless, there has been general acknowledgement of the higher immunological risk of pancreas transplantation. This has resulted in the evolution of strategies that employ more intense immunosuppressive protocols for pancreas transplantation compared with kidney transplantation. In Europe immunosuppressive protocols in solid-organ transplantation in general have been less aggressive compared with the USA. In the evolution of immunosuppression for pancreas transplantation tacrolimus has largely replaced ciclosporin starting from the mid-1990s. Similarly, mycophenolate mofetil (MMF) has replaced azathioprine in most immunosuppressive protocols (Fig. 9.6). There is a sound evidence base for this evolution. This evidence, reviewed below, has been provided not only by single-centre reports or registry analyses but also by prospective randomised trials. Figure 9.6 Immunosuppressive protocols used for maintenance in pancreas transplant recipients in the USA. US primary deceased donor pancreas transplants 1 January 2000–6 June 2004. CsA, ciclosporin; MMF, mycophenolate mofetil; SRL, sirolimus; TAC, tacrolimus. Data from the IPTR.
Pancreas transplantation
Introduction
Indications for pancreas transplantation
Pancreas transplantation from living donors
Patient selection for pancreas transplantation
Simultaneous pancreas–kidney transplantation (SPK)
Pancreas after kidney transplantation (PAK)
Pancreas transplantation alone (PTA)
Pancreas transplantation activity worldwide
The pancreas donor and the organ retrieval procedure
Pancreas retrieval operation
The pancreas transplant operation
Management of exocrine secretions
Management of the venous drainage
Immunosuppression in pancreas transplantation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree