12 Oral cavity, tongue, and mandibular reconstructions
Synopsis
 In head and neck reconstruction, a comprehensive review of the oral cavity, tongue and mandibular defects, the patient’s disease status, and overall condition and prognosis are critical to achieving optimal reconstruction results while minimizing complications. Evaluation of the defect, including its size, shape, geometry, and relationship to the available recipient vessels, should be thorough, and a strategic approach to flap selection and design should be taken, in order to reconstruct the oral defect accurately and restore the patient to his/her preoperative functional and aesthetic status.
In head and neck reconstruction, a comprehensive review of the oral cavity, tongue and mandibular defects, the patient’s disease status, and overall condition and prognosis are critical to achieving optimal reconstruction results while minimizing complications. Evaluation of the defect, including its size, shape, geometry, and relationship to the available recipient vessels, should be thorough, and a strategic approach to flap selection and design should be taken, in order to reconstruct the oral defect accurately and restore the patient to his/her preoperative functional and aesthetic status.
 There are several options to consider when performing a mandibular reconstruction, such as patient risk factors, defect characteristics, donor flap selection, and surgical technique. In mandibular reconstruction, the use of different tissue components to achieve composite reconstruction is essential for successful functional reconstruction. An understanding of the anatomical characteristics of the different osteocutaneous flaps available may increase the likelihood of selecting the appropriate donor flap for mandibular reconstruction.
There are several options to consider when performing a mandibular reconstruction, such as patient risk factors, defect characteristics, donor flap selection, and surgical technique. In mandibular reconstruction, the use of different tissue components to achieve composite reconstruction is essential for successful functional reconstruction. An understanding of the anatomical characteristics of the different osteocutaneous flaps available may increase the likelihood of selecting the appropriate donor flap for mandibular reconstruction.
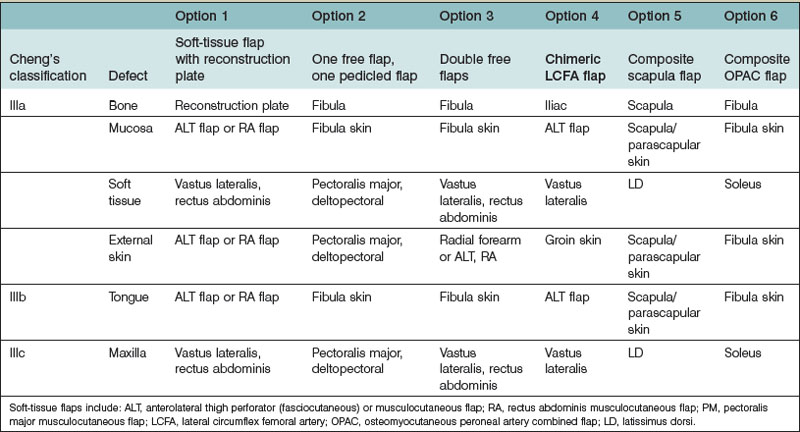
 For reconstruction of type III mandibular defects, several options exist, including the use of a soft-tissue flap with a reconstruction plate, one osteocutaneous flap with one pedicled flap, double free flaps, chimeric flaps, a composite scapular flap, and an osteomyocutaneous peroneal artery combined (OPAC) flap. The evolution of the fibula flap from a bone-only flap to an osteocutaneous flap and an OPAC flap may increase the clinical application of this flap for type III mandibular defects. Due to the triangular profile of the fibula, the placement of plates and screws on the lateral aspect of the fibula reduces the incidence of injury to its pedicle and septocutaneous perforators.
For reconstruction of type III mandibular defects, several options exist, including the use of a soft-tissue flap with a reconstruction plate, one osteocutaneous flap with one pedicled flap, double free flaps, chimeric flaps, a composite scapular flap, and an osteomyocutaneous peroneal artery combined (OPAC) flap. The evolution of the fibula flap from a bone-only flap to an osteocutaneous flap and an OPAC flap may increase the clinical application of this flap for type III mandibular defects. Due to the triangular profile of the fibula, the placement of plates and screws on the lateral aspect of the fibula reduces the incidence of injury to its pedicle and septocutaneous perforators.
Introduction
Reconstruction of the tongue is difficult due to its important role in articulation, deglutition, and airway protection.1 Among westerners, the tongue is the most common site in the oral cavity for primary cancers. The incidence of tongue cancer remains as high as buccal cancer due to the popularity of habitual betel nut chewing, smoking, and alcohol consumption in Asian countries. Any reconstructive attempt has to restore bulk and mobility in order for the tongue to be functional.
The goals of composite mandibular reconstruction include restoration of the bony scaffold, adequate oral continence and deglutition, obliteration of dead space, and re-establishment of optimal cosmesis. Dead space created by the extirpation of masticator muscles, the buccal fat pad, and the parotid gland can lead to fluid accumulation and infection. When soft-tissue losses are not adequately replaced following tumor resection, this can lead to a sunken appearance from soft-tissue contracture, trismus, plate exposure, and impaired speech and swallowing function.2 These conditions may be exacerbated by postoperative radiotherapy.
Historical perspective
Surgical treatment for head and neck cancer has been the standard of treatment since the 1940s. The concept of immediate reconstruction following tumor resection was introduced in 1951 and has been considered the gold-standard treatment for oral cavity cancers.3 However, surgical treatment at that time was for tumors with early stage, and the reconstruction was limited to small defects due to the inability to reconstruct a large defect. In the past, options largely followed the reconstructive ladder, with widespread use of primary closure, skin grafts, and pedicled flaps such as the infrahyoid myofascial flap,4 pectoralis major (PM) myocutaneous flap,5 or the trapezius islandized pedicle flap.6
Over time, many local and regional flaps were introduced to address the reconstruction of large head and neck defects after wide tumor resection of advanced-stage cancers. Among them, the deltopectoral flap, PM myocutaneous flap, and latissimus dorsi (LD) myocutaneous flap were the three major flaps originally utilized.7,8 However, their application was still limited. These flaps were limited by the length of the pedicle and the size of the flap. In addition, the requirement of a two-stage procedure and the less reliable blood flow at the very distal part of the flap make these flaps less desirable. In 1959, a free segment of jejunum was first transferred as a microsurgical flap for an esophageal conduit.9 This surgery introduced the concept of microsurgical free flap transfer, which was gradually popularized for head and neck reconstruction.9
The evolution of head and neck reconstruction gained speed after the concept of microsurgical free flap transfer became accepted.10,11 The first sensate fasciocutaneous free flap, a free sensate dorsalis pedis flap, was introduced in 1979.12 The free radial forearm flap was applied in oral cavity reconstruction.13 Microsurgical tissue transfer eliminates the requirement for a two-stage surgery. It also allows more freedom for flap inset to achieve better cosmetic and functional outcomes. In the early 1980s, free flaps were limited to axial fasciocutaneous flaps and myocutaneous flaps. The most commonly used free flaps were the radial forearm flap, rectus abdominis myocutaneous (RAM) flap,14 and latissimus dorsi (LD) myocutaneous flap. With the advancement of surgical instruments and techniques, better understanding of flap anatomy and vascularity, perforator flap dissection became possible. The concept of the freestyle free flap, based on any sizeable perforator, made more donor sites available for flap harvest, with less donor site morbidity. Nevertheless, there is not a single free flap that meets all reconstructive demands with regard to flap size, thickness, pliability, tissue bulk, and skin turgor. Choosing an optimal flap for reconstruction should be based on the clinical situation and the patient’s own preference. Currently, the radial forearm flap, RAM flap, latissimus dorsi flap and anterolateral thigh (ALT) flap are still the most commonly used flaps in buccal reconstruction. The preferred flaps vary between countries as flap selection is often influenced by cultural preferences and the patient’s body habitus.
Mandibular reconstruction initially involved the use of prosthesis, then gradually progressed into the usage of nonvascularized bone graft, tubed osteocutaneous skin flap, pedicled osteomusculocutaneous flap, and finally microsurgical osteocutaneous flap. Hausamen was the first surgeon to apply plates and screws for mandibular fractures in 1886.15 However, this was associated with high failure rates due to infections, plate exposures, plate fractures, wound dehiscence, and other complications. In 1976, Spiessl16 reported the use of metal plates to bridge a mandibular defect after tumor resection. Originally, plates were made of stainless steel; titanium plates are typically used today due to their biocompatibility. Kim et al. demonstrated that locking screws/plates, in combination with vascularized bone grafts, are associated with significantly fewer complications than nonlocking plates due to less compressive forces between the plate and the underlying bone.17 Despite major disadvantages,18 there are still indications for using plates for mandibular reconstruction in patients where medical conditions preclude a prolonged operation time or extended reconstruction procedures.
Freeze-dried allogeneic mandibular cribs with autologous iliac cancellous bone grafts were introduced by De Fries et al. for mandible reconstruction in 1971.19 A combination of autologous cancellous bone with fibrin glue was proposed by Tayapongsak et al. in 1994.20 Marx et al. also demonstrated the use of platelet-rich plasma gel as growth factor enhancement for these cancellous bone grafts.21,22
At the end of the 19th century, Sykoff23 performed a nonvascularized bone graft transplantation for mandibular reconstruction. He used a graft from the contralateral mandible to bridge a 4-cm mandibular defect. During World War I, this technique was used widely, with the tibia and rib serving as additional donor sites. In their book Gunshot Wounds of the Mandible, Klapp described various ways to bridge mandibular defects.24 Intermaxillary fixation and wired osteosynthesis were also used during that time. At present, many surgeons use corticocancellous block grafts from the anterior or posterior iliac crest. The survival rates of these grafts depend mainly on revascularization from the recipient site. Despite the possible complications such as bone resorption, partial bone loss, and pseudoarthrosis, nonvascularized bone grafts can be a good option for mandibular defects less than 5 cm in length.15,25
Regional osteomusculocutaneous flaps became popularized in the 1980s with the introduction of the PM myocutaneous flap in 1968 by Hueston and McConchie.26 Cuono and Ariyan27 reported the first pedicled rib graft with the PM myocutaneous flap. Robertson28 and Green et al.29 demonstrated the feasibility of using the PM myocutaneous flap with the sternum as a bone graft. Composite pedicled myocutaneous flaps transferred with clavicle bone graft have also been reported.15,30–32
Mowlem33,34 demonstrated the osteogenic potential of cancellous bone grafts in 1945. Autologous particulate bone cancellous marrow grafts showed a higher rate of osteogenesis and a lower rate of surgical complications compared to cortical grafts.35 Due to the lack of cohesion, the cancellous bone grafts were placed into different frames and cribs. Boyne36 began using metal cribs in 1969. Cribs of titanium, vitallium, tantalum, chrome cobalt, and stainless steel were used, with variable results. Modification of a titanium mesh system was reported by Tideman37 and Lee et al.38 in 1998 and 2009, respectively. Biodegradable poly(l-lactide) mesh was used successfully along with the cancellous bone graft by Kinoshita et al.39,40
The advent of vascularized bone grafts revolutionized mandibular reconstruction. McKee41 was the first to describe the use of a microvascular free rib graft for mandible reconstruction in 1978. Various donor sites for vascularized bone have been implemented successfully, including the metatarsal graft,42–44 radial forearm,45,46 scapula,47–49 iliac crest,48,50,51 and fibula bone.52,53
Taylor et al. first introduced the free fibula flap for clinical application in 1975.52 The reliability and anatomical study of the free fibula flap were well established by Wei et al.52,53 and Cho et al.54 The fibula flap was first applied for mandible reconstruction by Hidalgo55 and has now become the workhorse flap in vascularized bone replacement of mandibular defects.56–59 The reliability and consistent aesthetic and functional results with minimal bony resorption obtained using a free fibula flap were further confirmed by Cordeiro et al. at a 10-year follow-up.60
However, soft-tissue reconstruction is at least as vital as bone reconstruction in composite mandibular defects.61 Soft-tissue losses involve not only skin but also masseter muscle, buccal fat, and parotid glands in advanced-stage tumor resection. Wide tumor resection requires effective dead-space obliteration to prevent fluid accumulation, which results in postoperative infection and can compromise flap survival. The residual dead space can also result in soft-tissue contraction, which can significantly alter swallowing, chewing, speech, and aesthetic appearance. The fibula osteocutaneous flap is the first choice for mandibular reconstruction as it provides a long bony segment that can be safely osteotomized and can be transferred with a reliable skin paddle. For large defects where multiple tissue components are missing, more than one flap may be required for optimal reconstruction. A common choice is the fibula osteocutaneous flap in combination with the ALT musculocutaneous flap or RAM flap. In a selected group of patients, osseointegrated implants are offered for eventual dental implant placement. This can be performed during the primary operation in patients with mandibular defects resulting from the excision of benign lesions or trauma; otherwise, these procedures are performed secondarily. Although used less frequently, the iliac crest free flap and the scapula free flap are alternative options for reconstruction of composite mandibular defects. A new alternative to provide both bone and soft-tissue components as a combined flap for mandibular reconstruction is the OPAC flap, which is the modification of fibula osteospetocutaneous flap with inclusion of a cuff of soleus.62
Basic anatomy/disease process
Approximately 86% of all oral tumors are squamous cell carcinomas, while other tumors, such as verrucous carcinoma, sarcoma, melanoma, or lymphoma, occur less frequently. The mean age-adjusted incidence of oral cavity and phalanx cancer is 11.9 per 100 000 from 1975 to 2008 in the US; this value could be higher in specific areas where smoking and betel nut chewing are popular.63 In 2008, it was estimated that head and neck cancers comprised 2–3% of all cancers and accounted for 1–2% of all cancer deaths in the US.64,65 Most patients with head and neck cancer have metastatic disease at the time of diagnosis (with regional nodal involvement in 43% and distant metastasis in 10%). Moreover, patients with head and neck cancer often develop second primary tumors at an annual rate of 3–7%.66,67 The male-to-female ratio is currently 3 : 1 for the incidence of oral cavity and pharyngeal cancers.65
Statistical analysis of a 10-year period revealed a trend toward earlier diagnosis of head and neck cancer. Surgical treatment with or without radiation and chemotherapy remains the standard of care.68 Since the first introduction of the free intestinal flap in 195910 and the first fasciocutaneous free flap for head and neck reconstructions in 1976, free flap transfer has become the gold standard for reconstruction due to its high flap survival rate, improved cosmetic and functional results, and acceptable level of donor site morbidity.12 These techniques also facilitate resection of even more advanced but localized tumors.69,70
Inadequate oral hygiene and contamination may increase the risk of infection and also compromise the survival of any inadequately vascularized tissue.71 Irradiation produces detrimental acute and chronic effects not only in the periosteum and the marrow of the mandible but also in the oral mucosa and surrounding soft tissue.72–75 With chronic hypoxia, cellular and vascular damage lead to skin atrophy and increase the susceptibility to wound breakdown and decrease healing potential following minor trauma. Vascular changes initially occur in the microcirculation; however, with progression, larger blood vessels can be affected as well. Many of these issues are addressed by the transplantation of well-vascularized tissue with bone and skin components. Vascularized bone resists infection well, does not resorb, and is not dependent on the recipient bed vascularity for survival.76
Diagnosis/patient presentation
Currently, most patients with oral cancer still present with advanced-stage cancer.77,78 Most patients complain about oral ulcers, sore throat, a tongue mass, and pain with swallowing. Cuffari et al. demonstrated that the type of pain reported is related to the TNM staging of the tongue and mouth floor.79 A thorough physical examination, radiographic study of the tumor, and histology with TNM staging should be performed by both a surgical oncologist and a reconstructive surgeon prior to surgery. Any history of gunshot trauma, X-rays, and three-dimensional computed tomography (CT) scans of the defect should also be considered during preoperative planning. In addition to classical TNM staging,80 gene expression81,82 and profiling provide a subclassification based on DNA repair genes. This subclassification plays a role in predicting the clinical outcome after radiotherapy.83 Liao et al. addressed that upregulation of centromere protein H is correlated with poor prognosis and progression in tongue cancer patients.84 Chronic ulcer, leukoplakia, and tumor growth are regularly seen at specialized cancers.85 Visual loss as an initial symptom of squamous cell carcinoma of the tongue was published by Foroozan.86 It is important to keep in mind that rare constellations of symptoms may require differential diagnosis: a tumor or abscess of the tongue could also be a sign of atypical metastasis of lung cancer.87,88 Recently, a rare schwannoma of the tongue was reported by Cohen and Wang.89 Malignant fibrous histiocytoma of the tongue was reported by Rapidis et al.90
Patient selection and decision-making
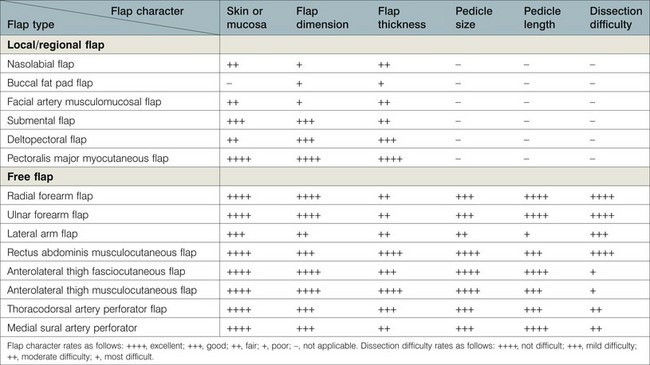
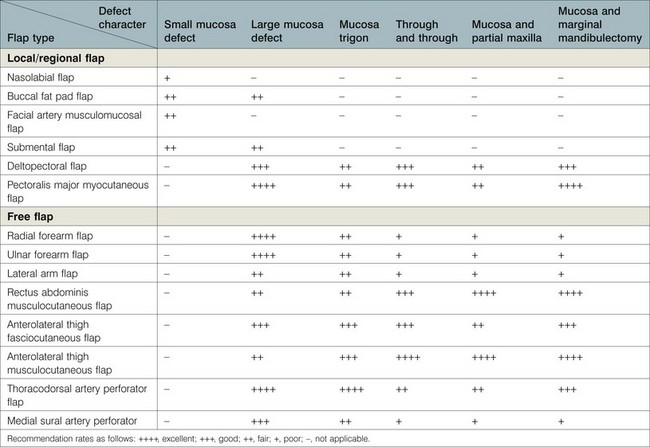
Most buccal mucosa, tongue, or mandibular defects result from cancer ablation surgeries. Before reconstruction, a comprehensive assessment of the defects, the patient’s general condition, and the availability of donor tissue should be performed. A thorough understanding of the missing tissue and its geometrical relation to each structure will elucidate the functional and aesthetic requirements of reconstruction and facilitate the selection of an optimal reconstructive method (Tables 12.1–12.3). The surgeon’s technique and decision-making ability as well as the patient’s general status contribute to the quality of the reconstruction.
Table 12.1 Comparisons of soft-tissue flaps for buccal mucosa and tongue reconstruction
Table 12.2 Selection of soft-tissue flaps for buccal mucosa reconstruction
Patient factors (Table 12.4)
Many oral cavity cancer patients have a history of smoking and alcohol consumption, which increases the risk of perioperative pulmonary and overall complications. These factors also affect the microvascular anastomosis in a free flap transfer.91,92 Diabetes mellitus is a risk factor for peripheral vasculopathy and is associated with a higher incidence of postoperative infection. A patient with end-stage renal disease undergoing a prolonged operation is at greater risk of developing postoperative fluid overload and other associated complications. Patients with Child’s class B or C cirrhosis had more complications, including pulmonary complications, acute renal failure, and sepsis, than those with class A cirrhosis (80% versus 19.1%).93 Advanced age is not a contraindication for extended-duration microsurgery. However, medical problems associated with chronological age, such as cardiopulmonary disease, atherosclerosis, and previous stroke, indicate a higher incidence of postoperative medical complications.86 These advanced oromandibular cancer patients are often malnourished, which has an impact on normal wound healing, pulmonary function, and postoperative recovery. Smoking should be ceased 2 weeks before a long operation to reduce pulmonary complications. For a malnourished patient, a short period of tube feeding before surgery can correct malnutrition status and improve wound healing and general recovery after surgery.
Table 12.4 Considerations in mandibular reconstruction
| Considerations | Details | |
|---|---|---|
| 1 | Patient’s risk factors | Smoking, old age, diabetes, malnutrition, cardiovascular disease, liver cirrhosis, local advanced disease, distal metastasis, recurrent or second primary cancer, postoperative radiation |
| 2 | Defects | Length and location of bone defects Size, volume, and components of soft tissue Radiated skin and vessels, previous scarring, cosmesis |
| 3 | Recipient vessels | Ipsilateral or contralateral |
| 4 | Selection of donor flaps | See Table 12.6 |
| 5 | Technique considerations | |
| Plating | Reconstruction plate or miniplates, preoperative three-dimensional computed tomography plating before or after pedicle division Occlusion with intermaxillary wiring | |
| Osteotomy | Lengths and number of segments Before or after pedicle division | |
| Flap inset | Before versus after anastomosis Bone inset first, then mucosa or external skin | |
| Microsurgical anastomosis | Artery first or vein first | |
| Osseointegration | Immediate/delayed Number of dental implants | |
Defect factors (Table 12.4)
The following are commonly applied reconstructive options.
Skin graft
Skin graft is a relatively easy procedure. However, its application in oral cavity reconstruction has largely been replaced by the use of pedicled and free flaps. The environment of the oral cavity is not conductive to survival of the skin graft. Furthermore, the progression of scar contracture from a skin graft often limits the mobility of the oral mucosa and tongue, which worsens the postoperative oral cavity function.94
Local/regional flap
Before the development of free-flap transfer, local and regional flaps were the treatment of choice. Today, the applications of pedicled flaps are limited for use in the reconstruction of small defects or in patients where free-flap transfer is not indicated (Tables 12.1–12.2).
Decision making for buccal reconstruction (Table 12.2)
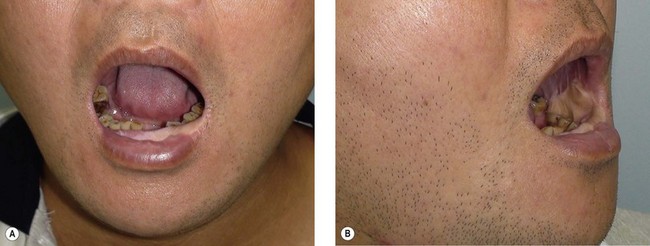
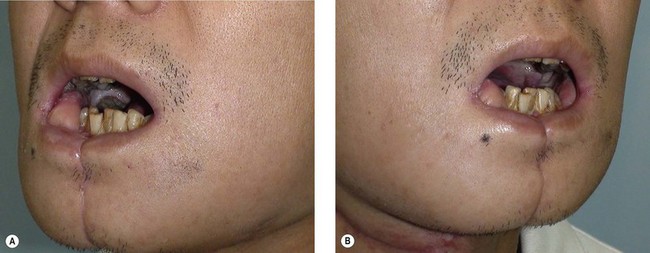
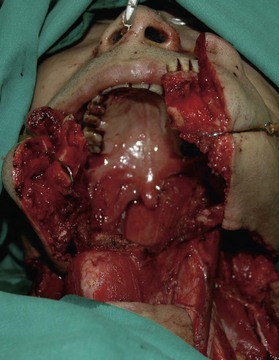
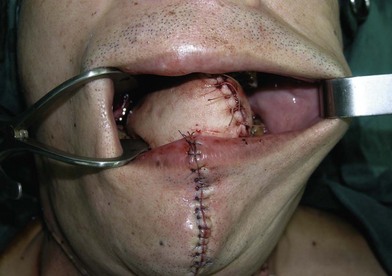
When the defect involves only the buccal mucosa, the reconstruction is relatively straightforward. The size of the defect should be measured with the mouth open at maximum. A mouth-opening retractor should be applied between the upper and lower teeth to facilitate adequate exposure. A sizeable flap is necessary to achieve satisfactory results; if the resurfacing is inadequate, an irreversible trismus will occur. A soft and pliable flap is generally the best choice for reconstruction. The pliability of the flap allows for better flap inset to fit the contour of the defect. Furthermore, it provides better functional restoration with regard to postoperative mouth movement, eating, speech, and facial expression (Figs 12.1–12.3).
When a buccal mucosa defect involves the buccal-gingival sulcus, the sulcus should be carefully recreated. The sulcus functions as a food reservoir during mastication and helps in directing the saliva and food toward the oropharynx during deglutition. In such cases, a slight folding of the flap is required to form the shape of the sulcus. It is also common that the inner surface of the lower and/or upper lip is involved (Figs 12.1 and 12.3). Although the wound between the edge of the lip and the lower gum can usually be closed primarily, direct closure results in an unnatural appearance and poor postoperative function.
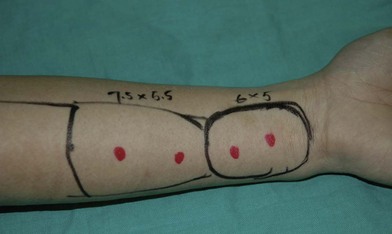
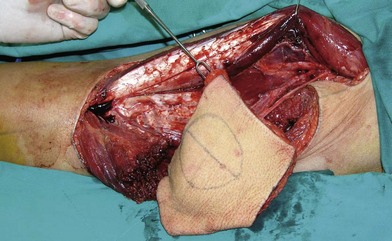
Flap selection for pure buccal mucosa reconstruction depends on the thickness of the flap required. According to the authors’ experience, a free radial forearm or ulnar forearm flap is usually adequate (Figs 12.4–12.7). A relatively thin ALT perforator flap is required for a thicker defect. A medial sural artery perforator (MSAP) flap is a feasible alternative.95
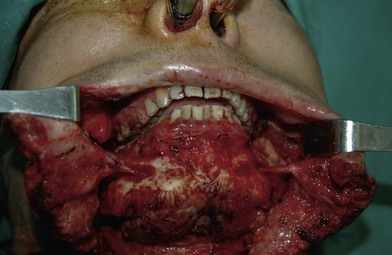
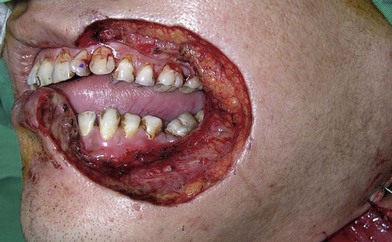
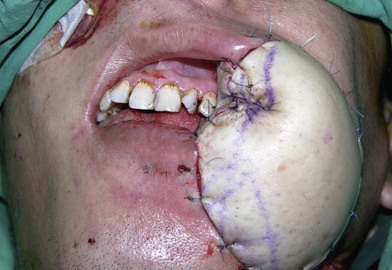
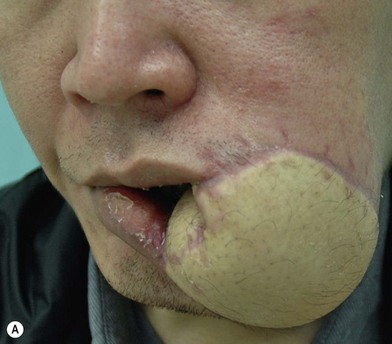
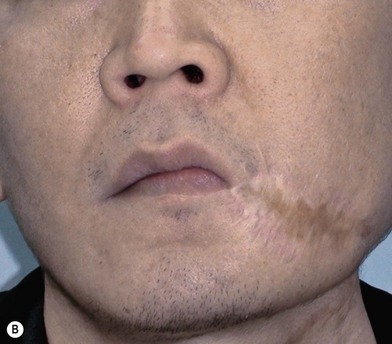
In severe trauma or in advanced buccal cancer resection, the defect can extend from the mucosa to the external skin (through-and-through defect), often requiring a bulky myocutaneous flap or a thick fasciocutaneous flap with de-epithelialization. Alternatively, a chimeric flap design with two separate skin paddles for intraoral and cheek reconstructions can achieve good aesthetic results (Figs 12.8–12.11). Each of the skin paddles can be customized according to the defect, preventing distortion of the mouth angle.
Table 12.1 summarizes the characteristics of each available flap and its clinical applications in buccal mucosa reconstruction. Table 12.2 provides guidelines for flap selection for variable buccal defects.
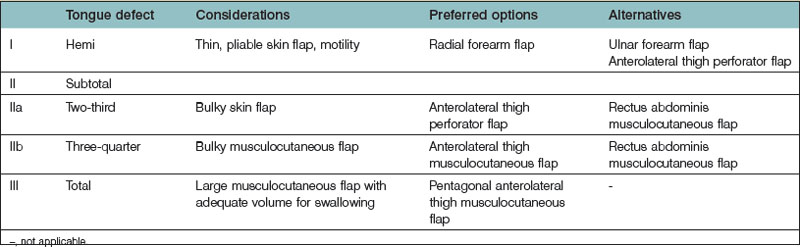
Decision making for tongue reconstruction (Table 12.3)
Reconstruction of the tongue is a difficult procedure due to its central role in articulation, deglutition, and airway protection.1 Several recent studies have used flap inset techniques to reconstruct a tongue with a three-dimensional shape closer to that of the actual tongue. Hsiao et al.96 described a radial forearm flap design with a narrow waist to recreate the omega-shaped profile of the tongue in cross-section. Chepeha et al.97 used a rectangular template, citing its simpler design and more dynamic reconstruction, as their method of choice. More recently, Davison et al.98 used a combination of native tongue tip rotation and wedge de-epithelialization in the flap for optimizing tongue tip sensation and to reduce pooling on the mouth floor, respectively. Chiu and Burd99 further expanded on this technique by describing their semicircular design with wedge de-epithelialization or resection to increase tongue elevation and deepening of the tongue floor at the mouth sulcus. Very little has been reported regarding the refinement of total tongue reconstruction, probably due to the mistaken notion that such reconstructions serve no purpose other than volume restoration.100,101
Most authors would classify tongue defects after tongue resection as hemiglossectomy, subtotal, and total glossectomy defects.96,97,102–106 A goal-directed classification for tongue defects should not only provide descriptions but also facilitate precise judgment with therapeutic consequences. Cheng’s modified classification (I, IIa, b, III) separates tongue defects into three major groups, which dictate the type of donor flap chosen (Table 12.3).107
Current reconstructive options either maintain mobility or provide bulk. Flaps that maintain mobility include the infrahyoid myofascial flap,108–110 MSAP flap,111,112 radial forearm flap,96,103,105,106,113–117 and ulnar forearm flap.118 Flaps that provide bulk include the rectus abdominis myocutaneous flap,119 latissimus dorsi myocutaneous flap,100 PM myocutaneous flap,5 and trapezius island flap.6 The ALT flap has emerged in recent decades as a popular option for head and neck reconstruction due to its reliability, long pedicle, and acceptable donor morbidity. Due to its versatility, this flap has been used both to provide bulk and to ensure mobility.97,103,106,114,120–130
Most publications have reported only the use of a single flap to reconstruct a limited range of tongue defects,100,101,116,118,125,131,132 while others compared two flaps but generally gave little or no information for why one flap was selected over another.103,106,111,116
For group I patients (defects ≤50%), a radial forearm flap is recommended because of its thinness, pliability, and long pedicle96,115,116,128,131,133–136 (Fig. 12.12); sometimes, the ALT perforator flap can be used instead in thin patients. Group II describes defects where up to 75% of the tongue is removed. In this classification system, a distinction is made between defects of 66% (IIa) and 75% (IIb). The additional division between groups IIa (66% resected) and IIb (75% resected) permits further refinements in flap selection. For group IIa patients (defects ≤66%), an ALT perforator flap is preferred over the radial forearm flap due to the larger flap size available, especially when encountering any accompanying mouth floor or buccal defects (Fig. 12.13). For group IIb defects, the small amount of remaining tongue (about 25%) probably has no functional role, but it likely plays an important role in maintaining the anatomical integrity of the base of the tongue, the retromolar trigone, or one side of the pterygoid fossa, depending on its location (Fig. 12.13 and Table 12.3). The thickness of the subcutaneous tissue in the ALT flap provides a better neotongue profile for defects crossing the midline in groups IIa and IIb.
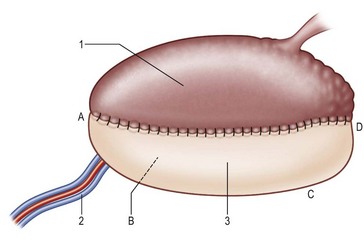
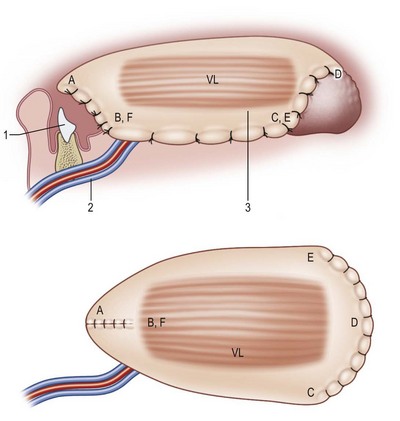
Fig. 12.12 The superior aspect of the tongue. Tongue defect type I (hemiglossectomy) is reconstructed with a thin pliable flap of rectangle shape (Table 12.3). A, tongue tip; B, floor of mouth; C, trigone; D, base of tongue; 1, native tongue; 2, radial artery and comitant veins; 3, radial forearm flap.
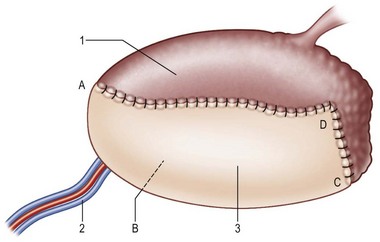
Fig. 12.13 Tongue defect type II (subtotal) is reconstructed with an anterolateral thigh fasciocutaneous or musculocutaneous flap (Table 12.3). A, close to tongue tip; B, floor of mouth; C, trigone; D, base of tongue; 1, native tongue; 2, lateral circumflex femoral vessel; 3, anterolateral thigh fasciocutaneous or myocutaneous flap.
In total glossectomy defect (group III), a specially designed pentagonal-shaped ALT myocutaneous flap facilitates better flap inset, provides adequate volume, and gives an aesthetically pleasing neotongue tip (Fig. 12.14). The “V” shape of the pentagon posteriorly allows a greater sloping profile when viewed in cross-section as well as an increasing posterior-to-anterior tongue projection. Such a design yields a well-shaped tissue bulk that resembles a normal tongue more closely in its lateral and frontal views. The anterior “I” shape allows increased elevation and freeing of the neotongue tip and also creates a gingival sulcus that prevents saliva pooling and subsequent drooling. Most of these patients provide ratings of “good” and above for diet or cosmetic appearance following reconstruction using this method (Figs 12.15–12.17).107
In both groups IIb and III, the ALT musculocutaneous flap rather than the fasciocutaneous flaps should be used for reconstruction to provide more bulk to augment the base of the tongue. The bulk at the base of the tongue is important to close off the oropharynx during swallowing with the assistance of the movement of hyoid bone. The rectus abdominis myocutaneous flap119 and the latissimus dorsi myocutaneous flap100,137 are alternative flap options for near-total or total tongue reconstruction, but with more significant donor site morbidity.
Decision making for mandibular reconstruction
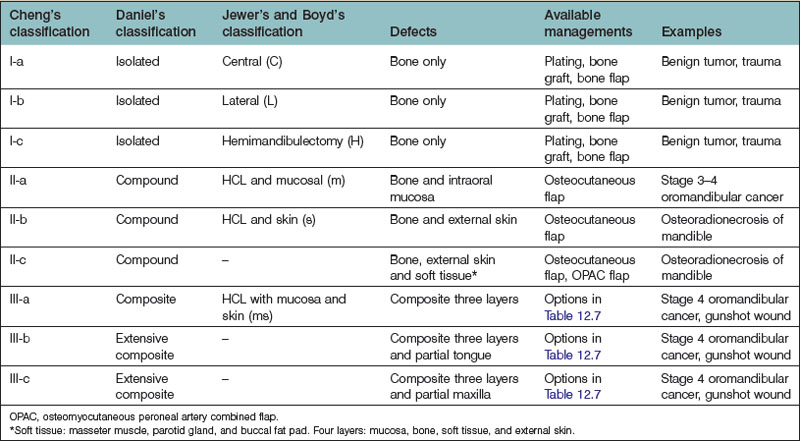
Daniel categorized lower jaw defects as isolated, compound, composite, extensive composite, or en bloc.138–140 Isolated defects include any single bone tissue resection; compound defects refer to those involving two tissue layers, such as bone and oral lining or bone and external skin. Composite defects indicate a three-layer-defect involving the mucosal lining, bone, and external skin; finally, extended composite or en bloc defects also include loss of soft tissue.138,139
Jewer et al. classified mandibular defects141 of the bone as central, lateral, or hemimandibulectomy. The classification system was modified by Urken et al.61 to consider associated soft-tissue defects and by Boyd et al.142 to recognize subcategories such as mucosa, skin, or a combination of both. These classifications were comprehensive and based on the availability of reconstructive options. With the development of microsurgical techniques, and better understanding of the perforator flap concept, more reconstructive alternatives became available. A modified mandibular defect classification is recommended by the authors to define further the components involved in the mandibular defect. This is outlined in Table 12.5. The available classifications for mandibular defects, together with examples and possible reconstructive options, are compared and summarized in Tables 12.6 and 12.7.
Treatment/surgical technique
Part I: Soft-tissue flaps
Local flaps
Submental flap
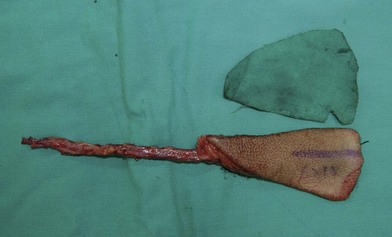
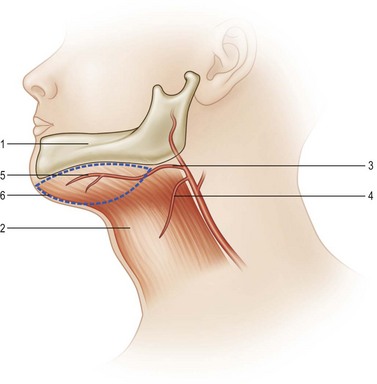
The blood supply of the submental flap derives from the submental artery, which is a continuous branch of the facial artery, located 5–6.5 cm away from the origin of the facial artery. This branch penetrates deep to the submandibular gland through the myelohyoid muscle below the mandible angle, extending medially deep to the anterior belly of the digastric muscle. As the vessel travels along the mandible margin, it sends off cutaneous perforators through the platysma muscle to the skin. The anatomy is constant, and the flow it provides to the submental skin is reliable.143,144
Flap design is initiated by marking the inferior mandible border as the upper flap margin (Fig. 12.18). The flap length extends from the ipsilateral mandible angle to the contralateral mandible angle. The flap width depends on the laxity of the skin: usually a width of 5 cm can be obtained. However, the flap can be even wider in older patients with loose skin. The flap can be elevated as an axial flap or a perforator flap. An easier surgical technique involves lifting the tissue as an axial flap without perforator dissection. In that case, an incision can be made in the inferior margin of the flap directly through the platysma muscle. Then, the dissection is carried out with division of the anterior belly of the digastric muscle, which is included in the flap to ensure inclusion of the submental perforator. When the pedicle is identified, its branches to the submandibular gland should be ligated carefully. Finally, when reaching the inferior border of the mandible, care should be taken not to injure the marginal mandibular nerve. The pedicle is then skeletonized and the flap is ready to be transferred. If the flap is going to be transferred as a free flap, dissection of the pedicle can be continued to the facial vessels to obtain a better size and length for anastomosis.143–145
The arc of the distal-based submental flap is suitable for rotating it to the lower third of the face and the entire oral cavity, making it a suitable pedicle flap for oral cavity reconstruction.145,146 The only drawback is that many oral cavity reconstructions are performed during cancer ablation surgery, during which neck lymph node dissection is often required. After a neck lymph node dissection, the continuity of the submental skin and its main pedicle are usually disrupted (Tables 12.1 and 12.2).
Regional flaps
Deltopectoral flap
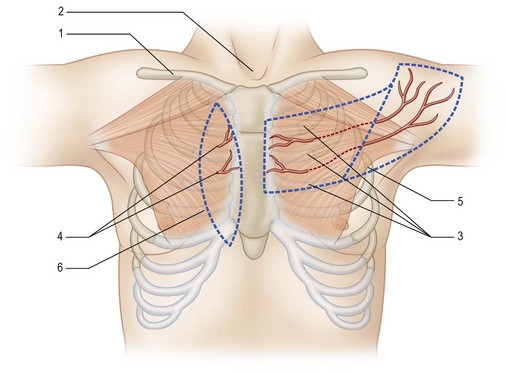
The deltopectoral flap was popularized around 1965 by Bakamjian.7 It is located in the anterior chest wall. Based on the internal mammary perforators in the second and third intercostal spaces, the flap extends from the central chest wall to the deltoid region.
The flap can be designed around the perforators, which can be located at the second and third intercostal spaces using a pencil Doppler. The flap base is situated in the anterior chest wall, and the flap extends superolaterally to the deltoid region. The exact flap required should be measured to ensure its ability to reach the defect. For an oral cavity reconstruction, a lengthier flap is usually required. However, the distal flap is a random flap with an uncertain blood supply. To obtain a longer flap, a prefabrication or a delayed procedure is usually required to reduce the risk of distal flap necrosis (Fig. 12.19).147
The disadvantages of the deltopectoral flap include the unattractive donor site scar, the requirement of a second surgery for flap division, and the possibility of a delayed procedure to lengthen the available flap. Today, the deltopectoral flap has been largely replaced by the PM myocutaneous flap (Tables 12.1 and 12.2).
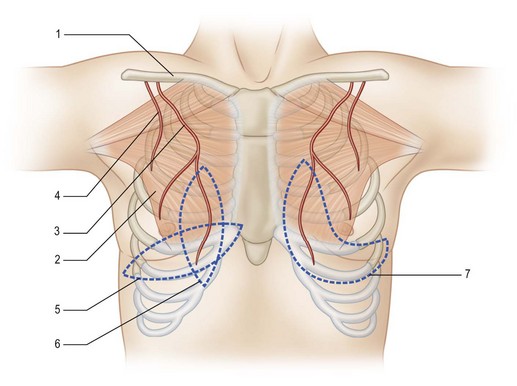
Pectoralis major myocutaneous flap
Since its introduction by Ariyan in 1979, the PM flap has gained in popularity.8 The PM flap has a reliable blood supply that provides a large skin paddle with sufficient bulk tissue for large-size reconstruction. This flap can reach the neck and lower third of the face, making it practical for intraoral and external cheek reconstruction. Today, the PM myocutaneous flap is useful for salvage procedures and in a vessel-depleted neck where a free flap transfer is not indicated.
The PM flap is a myocutaneous flap comprising the PM muscle and its overlying skin. The blood supply of the PM muscle mainly comes from the thoracoacromial artery and parasternal perforators. The lateral thoracic artery runs along the lateral edge of the PM muscle and sends off branches to augment the circulation of the PM muscle. The thoracoacromial artery runs inferiorly at the midpoint of the clavicle and this can be used as a pivot point when designing the flap (Fig. 12.20).