Fig. 27.1
(a) Skin island designed over the lateral aspect of the rat region. (b) Cutaneous perforator of iliolumbar vessels. (c, d) Schematic presentation of skin island and muscle component of IBOMC flap
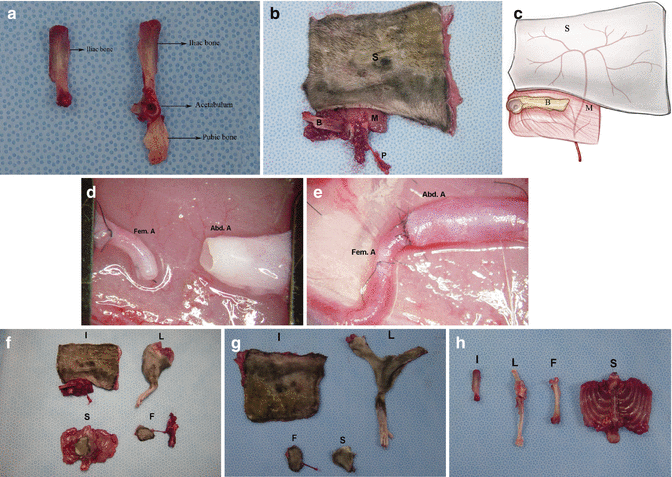
Fig. 27.2
(a) Comparative view of sizes of whole iliac bone and hip bone. (b, c) The composite iliac osteomusculocutaneous flap isolated on the vascular pedicle. (d, e) View of vascular pedicle and recipient’s arteries before and after anastomosis. (f) Composition of different vascularized bone marrow transplantation models. (g) Comparison of skin components of vascularized bone transplant models. (h) Harvested bone components of VBMT SVS, superior ventral spine of iliac bone. Ib iliac bone, Hb hip bone, S skin component, M muscle component, B bone component, P vascular pedicle, Fem. A femoral artery, Abd. A abdominal aorta, I iliac osteomusculocutaneous allograft model, L limb allograft model, S sternum allograft model, F femur allograft model
Four IBOMC grafts were elevated on the vascular pedicle in Lewis donors (RT1l) and were transferred to the groin region of Lewis (RT1l) recipients. In two rats, graft pedicles were anastomosed to the femoral vessels while the remaining two grafts (n = 2) were transferred to the groin region of Lewis (RT1l) recipients as composite grafts without pedicle anastomosis as a control for non-vascularized graft survival. All animals were observed until day 21 post-transplant.
Next, anatomic dissection was performed in LBN (RT1l + n) rats (n = 2) each for limb [10], sternum, and vascularized femoral bone models [11] to compare the size of skin components and number of BM cell population in the bone compartment of these models.
Following measurements were taken (Fig. 27.2).
1.
Measurements of skin surface area: After dissection, all skin components were harvested from VBMT models, and the total cross-section area of skin was measured using millimetric graph paper (Fig. 27.2).
2.
Evaluation of BM cell population: To compare BM cell populations in different VBMT models, iliac bone, femur, sternum and limb bones were harvested. To simulate the vascularized BM load of the limb transplant, half of the femur and intact tibia (a bone component of limb allograft) were resected from the limb transplant (Fig. 27.2). BM cells of the iliac bone, femur and sternum were counted using the trypan blue exclusion technique.
IBOMC Transplant Groups
Two subgroups of transplantation were studied. Isograft transplants were performed between Lewis (RT1l) rats (n = 12). Iliac osteomusculocutaneous grafts were elevated as described above. In the recipient rat, the left femoral vessels were exposed and dissected up to the inguinal ligament proximally and down to the branching from the superficial epigastric vessels distally. Keeping long pedicle, the femoral artery and vein were ligated and transected before their bifurcation at the popliteal region. The groin skin was resected from the recipient rat to make the defect proportional to the donor flap size. The abdominal aorta and iliolumbar vein of the isograft donor pedicle was anastomosed with the femoral artery and vein of the recipient using the standard end-to-end microsurgical technique with 10-0 nylon sutures (Fig. 27.2).
After the vessels’ patency was confirmed and hemostasis completed, the bone and muscle component of the graft was placed in the inguinal space of the recipient in an oblique position, parallel and inferior to the inguinal ligament. The skin component of the graft was sutured to the edges of the previously created skin defect in the groin region of recipient with absorbable suture material (4/0 Vicryl Ethicon). In the allograft transplant group, iliac osteomusculocutaneous grafts were transplanted from LBN (RT1l + n) (n = 6) donors to Lewis (RT1l) (n = 6) recipients using the same technique described above. Following transplantation, immunosuppression protocol of cyclosporine A monotherapy was used with dosage of 16 mg/kg/day in the first week, tapered to 8 mg/kg/day during the 2nd week, to 4 mg/kg/day during the 3rd week and to 2 mg/kg/day during the 4th week and maintained at this level thereafter.
Assessment of Transplant Viability
Direct Observation
Viability of the skin component of isograft and allograft transplants of the IBOMC graft was followed by daily observation. The survival of the skin island was evaluated during postoperative follow up by gross inspection of flap color, edema, erythema, hair loss, desquamation of the overlying skin, bone exposure, and necrosis.
Microangiography
Microangiography was performed to delineate the preservation of vascular territories of the transplant in two animals in the isograft transplant groups on post-transplant day 15. After cannulation of the common carotid artery, 50 cc of the mixture (at 70 °C) 50 g of silver oxide, 5 g of gelatin, and 100 cc of the 0.9 % sodium chloride was injected with a syringe. The grafts were stored at 4 °C for 4 h and underwent radiography with a soft X-ray machine, using Microvision-C mammography film.
India Ink Injection Studies
In two isograft transplants, viability of flaps was evaluated by the presence of dye in the vessels after India ink injection at post-transplant day 15. Following cannulation of the common carotid artery with a 24-gage catheter, 5 ml of India ink was injected. The graft was harvested and the bone segment was decalcified using commercial decalcifying solution (Protocol decalcifier A; Fischer Scientific, Pittsburgh, PA, USA) for 3 days. All graft components were stained with hematoxylin and eosin (H&E), and examined under light microscopy for the presence of India ink in blood vessels of the skin, muscle and in the BM and cortex of iliac bone.
Histology
On post-transplant day 200, animals were euthanized; isograft (n = 2) and allografts (n = 6) were harvested; skin and muscle components were stored in 10 % buffered formalin; and paraffin sections were stained with H&E for evaluation. The bone components were decalcified and sections from the iliac bone were processed and stained routinely. Each slide was evaluated by a pathologist blinded to the study for viability of skin, muscle and bone; for the presence of hematopoietic cells; and for signs of fibrosis and rejection.
Immunologic Evaluation
Flow Cytometry Analysis
Flow cytometry (FC) established the presence of donor-specific chimerism for donor MHC class I (RT1n) antigen in the peripheral blood of Lewis recipients during observation time at 7, 21, 63, 100 and 150 days post-transplant. Chimerism was assessed by a combination of conjugated mouse anti-rat mAb RT1n-FITC (for MHC class I of donor cells RT1n, clone MCA156; Serotec Ltd, Oxford, UK) with mAb specific for T cells: CD4−PE (clone OX-35), CD8a−PE (clone OX-8), B-cell CD45RA−PE (clone OX-33) and monocytes, granulocytes CD11b/c −PE (clone OX-42) (BD Bioscience Pharmingen Inc., San Diego, CA, USA). After incubation, samples were lysed and fixed with 1 % PFA solution. Negative control panels were tested and included isotype-matched antibodies (IgG1−FITC/IgG2−PE) and phosphate buffered saline samples. Analysis was performed on 1,104 cells using facs scan (BD Bioscience Pharmingen Inc., San Diego, CA, USA) and Cell Quest software.
To evaluate donor cell engraftment into Lewis (RT1l) BM compartment, BM samples were harvested from the recipient contralateral bone by aspiration biopsy and evaluated by flow cytometry using mouse anti-rat mAb RT1n-FITC (for donor MHC class I in combination with mouse anti-rat mAb CD90PE (BD Bioscience Pharmingen Inc.) as described above.
Immunohistochemical Analysis
The migratory potential of donor-specific cells into lymphoid organs of IBOMC allograft recipients was evaluated using mAb specific for donor MHC class I. Lymph nodes, spleen, and thymus were harvested from the euthanized representative rats at day 200 post-transplant. Samples were immediately snap-frozen in liquid nitrogen, cut (4 lm), and fixed for 10 min in acetone, air-dried, and blocked for endogenous peroxidase before incubation with mouse anti-rat RT1n (for MHC class I) mAb for 30 min at room temperature. The binding of primary antibodies was detected using a DAKO LSAB2 System (DAKO, Carpinteria, CA, USA), peroxidase, and aminoethylcarbazole (AEC) in accordance with the manufacturer’s instructions. Slides were counterstained in hematoxylin and mounted in Faramount. Sections incubated with nonspecific IgG were used as negative control.
Results
Anatomic dissection studies established a repeatable anatomic pattern in all operated animals and revealed that the iliolumbar artery, which was found to supply the IBOMC graft, originated from the abdominal aorta caudal to the origin of the renal artery, whereas the iliolumbar vein originated from the inferior vena cava at approximately the same level. The iliolumbar artery branched into the lumbar and iliac branches, and during its course a small arterial branch directly supplied bone which branched off before leaving the abdominal cavity at the level of the upper and lateral border of the iliac bone. This branch, penetrating the skin, supplied the flank and hip regions.
In the anatomic study group, at day 21 post-transplant, large composite grafts without vascular supply became totally necrotic whereas, as expected, all skin islands of the IBOMC graft were viable. Histologic examination of the skeletal muscle and bone segments showed skeletal muscle cells, which appeared normal and viable osteocytes within the bony trabeculae. However, examination of the graft group without vascular supply revealed nonviable, necrotic skeletal muscle cells and lacunae lacking osteocytes (Fig. 27.3).
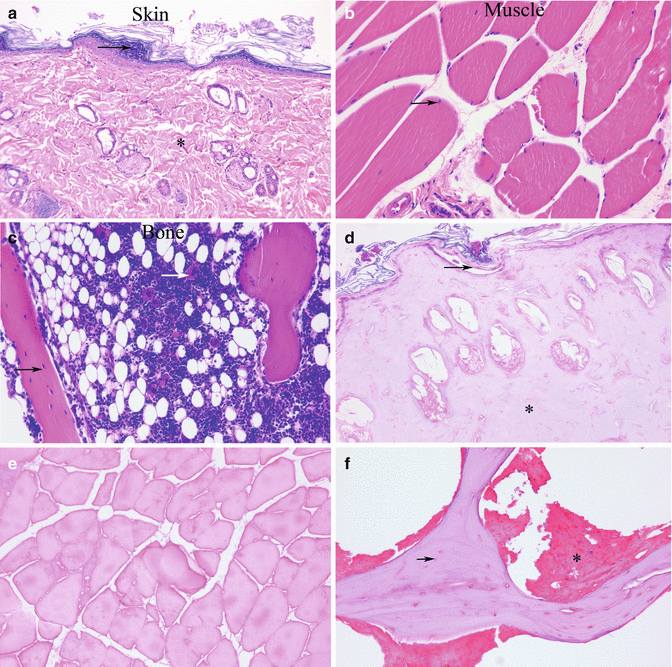

Fig. 27.3
Histologic sections of isograft Lewis (RT11) demonstrate viability of transplant components at day 21 post-transplant. (a) Intact epidermis (black arrow) and dermis (Hematoxylin-eosin stain; original magnification ×50). (b) Viable myocytes in the skeletal muscle components. Multiple nuclei (black arrow) lie at the periphery of the muscle fibers (Hematoxylin-eosin stain; original magnification ×400). (c) Viable bone trabeculae and bone marrow components. Osteocytes in lacunae (black arrow), hematopoietic cells and megakaryocytes (white arrow) (Hematoxylin-eosin stain; original magnification ×200). Histologic sections of composite isografts (Lewis [RT11]) without vascular supply show necrotic and disturbed components at postoperative day 21. (d) Diffuse necrosis of the epidermis (black arrow) and dermis (Hematoxylin-eosin stain; original magnification ×200). (e) Nonviable, necrotic skeletal muscle cells without nuclei (Hematoxylin-eosin stain; original magnification ×200). (f) Necrotic bone segment with empty lacunae (black arrow) and a disturbed and necrotic bone marrow component lacking hematopoetic cells (Hematoxylin-eosin stain; original magnification ×400)
Comparison of the IBOMC transplant with other VBMT models is presented in Table 27.1. When weights of CTA/VBMT models were compared, limb transplant was found to be the heaviest graft (18.84 g), followed by iliac (15.70 g), sternum (14.80 g), and vascularized femoral bone (2.4 g) graft. Measurements of the skin island surface area based on the diameter of different VBMT models revealed the following surface area values: IBOMC, 62.96 cm2; limb transplant, 24.12 cm2; sternum, 5.4 cm2; and skin component of vascularized femoral bone, 5.04 cm2.
Table 27.1
Comparison of iliac, limb, femur and sternum VBMT models for size of skin component, weight of the graft, and number of bone marrow cell populations within the bone compartment
VBMT models | Skin surface area (cm2) | Weight (g) | Bone marrow cell populations (106) |
|---|---|---|---|
Iliac | 57.96 | 15.70 | 25 |
Limb | 24.12 | 18.84 | 48.75 |
Femur | 5.4 | 14.80 | 50 |
Sternum | 5.04 | 2.4 | 7.5 |
The count of BM cell numbers in VBMT models revealed the following values from highest to lowest cell counts:→ vascularized→ femur,→ 50 × 106;→ limb→ (including tibia and a half femur), 48.75 × 106; iliac bone, 25 × 106; and sternum, 7.5 × 106 (Table 27.1).
Clinical Assessment of IBOMC Transplants
Direct Observation
During follow-up period up to 200 days post-transplant, skin islands of all flaps in isograft (n = 2) and allograft (n = 6) transplant groups were completely viable (Fig. 27.4a).
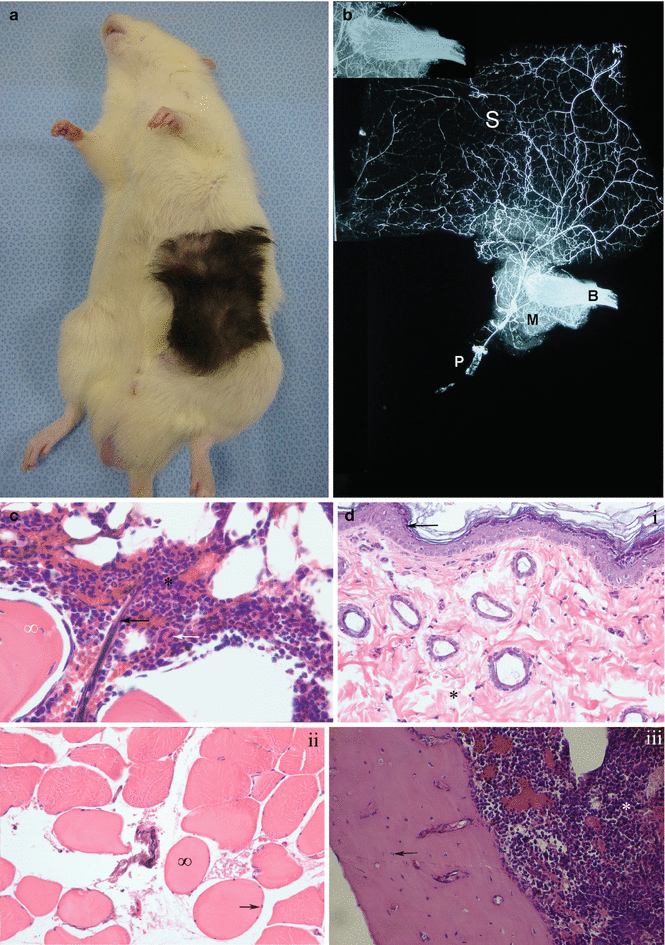

Fig. 27.4
(a) Assessment viabilities of recipients of IBOMC allograft Lewis (RT11) from LBN (RTl1+ n) donor at post-transplant day 150. (b) Microangiographic analysis of isograft transplant demonstrates the course of the pedicle. The vascular filing with contrast material is depicted in the bone (small picture at the corner of frame). S skin, M muscle, B bone, P pedicle. (c) Histologic examination of the bone marrow and bone trabeculae from the isograft transplant demonstrates perfusion of the bony component, evidenced by the presence of ink in vessels (black arrow) at post-transplant day 15. Hematopoietic cells and megakaryocytes (white arrow) within the bone marrow and osteocytes within the bone trabeculae (∞) (Hematoxylin-eosin stain; original magnification ×400). (d) Histologic sections of vascularized isograft (LBN [RT 1 + n]) demonstrate viability of the transplant components at post-transplant day 200. (i) Normal epidermis (black arrow) and dermis (Hematoxylin-eosin stain; original magnification ×50). (ii) Nuclei (black arrow) and healthy fibers (∞) in intact skeletal muscle cells (Hematoxylin-eosin stain; original magnification ×400). (iii) Viable osteocytes within lacunae (black arrow) and hematopoietic cells within the bone marrow. (Hematoxylin-eosin stain; original magnification ×200)
Microangiography
Microangiograpic study revealed preserved vascular territories of all components of the grafts, including skin, muscle, and bone compartment (Fig. 27.4b).
India Ink Injection Studies
Dye study with India ink demonstrated ink uptake by the vessels in the BM and cortical bone, confirming bone perfusion via the vascular pedicle (Fig. 27.4c).
Histologic Examination
Day 200 post-transplant, histologic examination of IBOMC allotransplants did not reveal any signs of rejection, such as blood cell extravasation, edema, necrosis or pigment incontinence. The normal architecture of the skin, skeletal muscle and bone was preserved, and the skeletal muscle cells and osteocytes within the bone trabeculae were viable (Fig. 27.4d).
Immunologic Evaluation
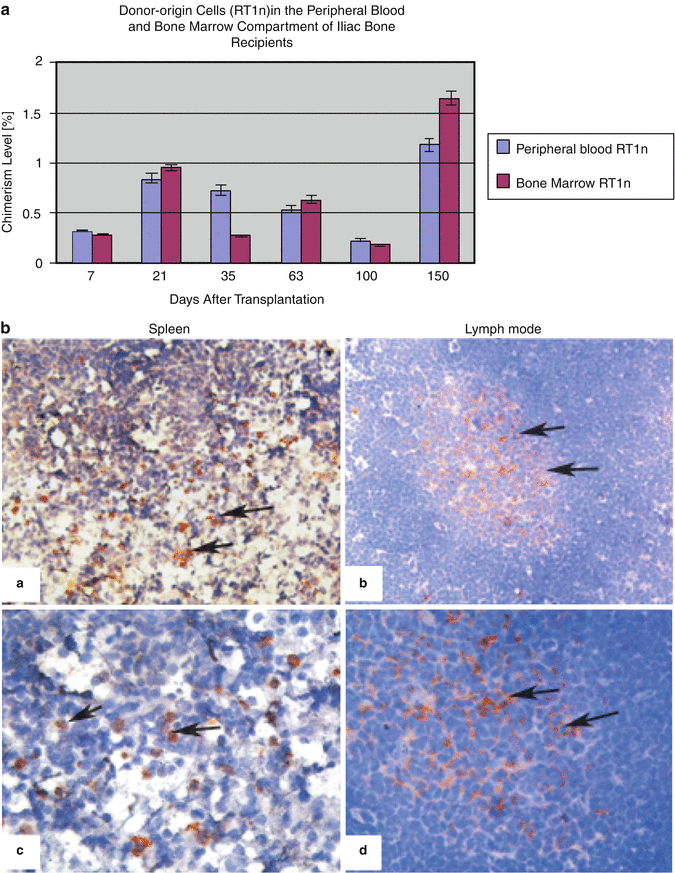
Donor chimerism was evaluated as a sum of donor-origin T-lymphocytes (RT1n/CD4, RT1n/CD8), B-lymphocytes (RT1n/CD45RA) and granulocytes/monocytes (RT1n/ CD11b/c). The kinetics of chimerism is represented as the total chimerism level of donor-origin cells (RT1n) in the peripheral blood and BM compartment of recipients.
Donor-specific chimerism in peripheral blood of IBOMC allograft recipients was assessed below 1 % up to 100 days post-transplant, and at 1.2 % at 150 days posttransplant.
Donor chimerism in the BM compartment of IBOMC allograft recipients was assessed below 1 % at 100 days post-transplant. By contrast, at day 150 post-transplant, higher chimerism was confirmed by the presence of 1.65 % of donor-origin RT1n cells in the recipient BM compartment. Comparison of donor-derived chimerism in BM and peripheral blood of recipients is illustrated in (Fig. 27.5a).
Get Clinical Tree app for offline access