Neurobiology of the Skin: Introduction
|
The Nervous System and Skin
This chapter discusses the structural basis and the specific molecules involved in the interactions between the skin and different portions of the nervous system.
The peripheral nervous system provides essential information to the rest of body during injury of “danger signals” such as parasites, UV radiation, toxins, allergens, pH changes, or “stress”. This information can be modulated at various levels including the brain, spinal cord, dorsal root ganglia (DRG), peripheral sensory nerve endings, autonomic nerves and neurons, etc; and through specialized structures like Pacini bodies or specialized cells such as Merkel cells. This closely woven group of structures and their molecules are ultimately and critically involved in normal cutaneous biology and skin diseases (eTable 102-0.1). In conjunction with the spinal cord and the brain, peripheral sensory nerves have afferent functions; their endings detect physical stimuli such as touch, heat or cold, and chemical mediators into the skin from nerve endings and also have efferent functions in the skin. (eFig. 102-0.1). These sensory nerves critically contribute to skin development before birth and to protection and homeostasis after birth. In addition, autonomic nerves modulate both physiological and pathophysiological functions as part of the stress response to external or endogenous stimuli, and form a vital link communicating with the vascular, endocrine, and immune systems (eTable 102-0.1).
Physiological Function | |
Vasodilatation Vasoconstriction Body temperature Perception of heat, cold, chemicals, toxins, touch, microbial agents, pressure, vibration (pain withdrawal and scratching as physiological responses to noxious stimuli) Antigen presentation Pigmentation Keratinocyte growth and differentiation Regulation of muscle cells (erector pili muscle, sweat glands) Regulation of hair follicle growth and apoptosis | |
Pathophysiological Implication | |
Amyloidosis | Pruritus, gene defect in neuronal cytokine receptors |
Atopic dermatitis | Pruritus, neurogenic inflammation, cytokine regulation |
Contact dermatitis | Pruritus, burning pain, immunomodulation |
Prurigo | Induction, aggravation of pruritus |
Urticaria | Pruritus, mast cell degranulation, dysregulation of neuronal receptors (heat, cold, mechanical?) |
Rosacea | Erythema, edema, tingling, burning, pain |
Psoriasis | Pruritus (30% of patients), stress-induced exacerbation? |
Lichen planus | Pruritus |
Herpes zoster | Neuropathic pain |
Pigmentation disorders | Regulation of melanocytes |
Scleroderma | Vasoconstriction, recruitment and activation of mast cells? |
Notalgia paresthetica | Neuropathic pruritus, nerve entrapment |
Brachioradial pruritus | Localized neuropathic pruritus, nerve impingement |
Pruritus | See Chapter 103 |
Pain | Stinging, burning, tickling, hypernociception, allodynia |
Psycocutaneous disorders | Dysesthesia, pain, burning, parasite tickling |
Drug eruption | Pruritus, burning |
Wound healing | Cell regulation, delayed wound healing, diabetic Neuropathy, immune defense |
Stress-induced hairloss | Hair follicle apoptosis, cell cycle?, exacerbation/aggravation of alopecia areata ? |
Autoimmune disease | Arthritis, myositis |
Burning mouth syndrome | Burning pain |
Hemorrhoids | Pruritus, neuropeptide release |
Hyperhidrosis | Neuronal regulation of secretion and muscle cells |
Acute photodermatitis | Burning pain, CGRP release, inflammation |
Neurofibromatosis | Pain, proliferation |
Raynaud phenomenon | Exaggerated vasoconstriction and vasodilatation |
eFigure 102-0.1
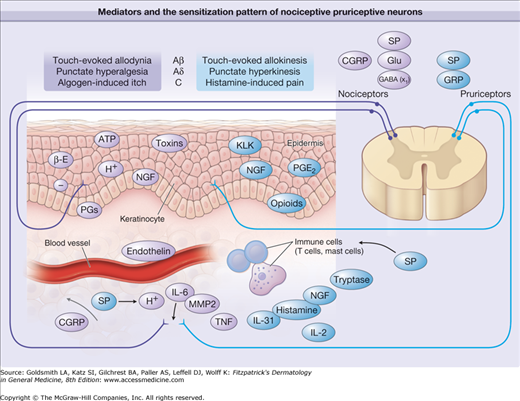
Mediators and the sensitization pattern of nociceptive and pruriceptive neurons. Sensitizing and activating mediators in the skin are shown for primary afferent fibers involved in itch and pain processing. Predominantly pruritic mediators are shown in red, algogenic mediators are shown in blue, and mediators equally involved in pain and itch are shown in yellow. Note that different classes of fibers subserve pain (mechano-sensitive polymodal nociceptors and mechano-insensitive “sleeping” nociceptors) and itch (histamine-sensitive mechano-insensitive pruriceptors4 and probably mechano-sensitive pruriceptors). In the spinal cord, noxious input can induce central sensitization for pain, and pruriceptive input can provoke central sensitization for itch. Of note is the corresponding pattern of central sensitization to touch by amyloid-β (A-β) fibers (allodynia vs. allokinesis), by A-δ fibers (punctate hyperalgesia vs. punctate hyperkinesis), and by C fibers (histamine-induced pain vs. algogen-induced itch). x1 = inhibitory interneurons ACh = acetylcholine; ATP = adenosine triphosphate; β-E = β-Endorphin; CGRP = calcitonin gene-related protein; GABA = γ-amino butyric acid; Glu = glutamate; GRP = gastrin-releasing peptide; H+ = hydrogen ion; IL = interleukin; KLK = kallikrein; MMP = matrix metallo proteinase; NGF = nerve growth factor; PGE2 = prostaglandin E2; SP = substance P; TNF = tumor necrosis factor.349
Sensory as well as autonomic (in the skin predominantly cholinergic sympathetic) nerves influence a variety of physiologic and pathophysiologic functions of the skin such as embryogenesis, vasoconstriction, vasodilation, body temperature, erector pili movement, regulation of the function of the pilosebaceous unit, sensing physical, chemical and biological stimuli on the skin surface; modulating epidermal barrier function, cell secretion, cell growth and differentiation, cell nutrition and apoptosis, nerve growth, inflammation, immune defense, and wound healing, respectively (eTable 102-0.1).
In unstimulated cutaneous nerves, neuromediators are stored in cytoplasmic vesicles. On direct or indirect activation through physical, mechanical, electrical, chemical or biologic or endogenous inflammatory processes lipids, cytokines or neurotrophins (NT), a significant increase of regulatory neuropeptides, or oxygen products can be detected at the site of inflammation within seconds to hours. Thus, mediators derived from sensory or autonomic nerves may play an important regulatory role in the skin under many physiologic and pathophysiologic conditions. However, in addition to neuroimmunomodulation in the periphery, a subtle and complex communication network also exists between the spinal cord, the central nervous system (CNS), and the immunoendocrine system that also modulates skin function. In addition, neuropeptides and NTs can be upregulated and released by nonneuronal cells under certain circumstances that may amplify or counterregulate the neurogenic stimulus. While certain neuropeptides [e.g., substance P (SP)] exert clear-cut proinflammatory effects during inflammation, others such as calcitonin-gene-related protein (CGRP) may be acutely released to induce vasodilatation (proinflammatory), but in addition counterregulate inflammatory responses like antigen presentation and immunosuppression in order to reestablish skin homeostasis at the later stage of inflammation.
The skin expresses a variety of receptors for these neuromediators, such as G protein-coupled receptors, ion channels and certain cytokine receptors (see Tables 102-1 and 102-2). This neuromediator–receptor interaction is controlled by endopeptidases [neutral endopeptidase (NEP), angiotensin-converting enzyme (ACE), endothelin-converting enzyme (ECE)], which terminate neuropeptide-induced inflammatory or immune responses.1–3 A close multidirectional interaction between neuromediators, high-affinity receptors, and regulatory peptidases is critical to maintain or reestablish tissue integrity and regulating pathophysiologic conditions in the skin. Ion channels are promiscuous and can be activated by physical stimuli (heat, cold), chemicals (e.g., capsaicin, menthol, protons) as well as lipid metabolites like prostaglandins. Activation of ion channels by cannabinoids (CB) has been also recently described.
Pruritogenic Stimuli (In Alphabetic Order) | Receptors | Sources, Receptors by which Expressed | Comments |
|---|---|---|---|
Acetylcholine (ACh) | Nicotinergic (nAChR) and muscarinergic (mAChR) ACh receptors | Autonomic cholinergic nerves, keratinocytes, lymphocytes, melanocytes, dermal fibroblasts, endothelial cells | Mediates itch in atopic dermatitis; mAChR3 is probably involved in itch. |
Calcitonin gene-related peptide (CGRP) | CGRP receptors | Sensory nerve fibers | Expressed on central terminals; sensitizes receptive endings. Associated with increased pain transmission, prolongation of itch latency after substance P injection (inhibitory effect on itching). Involved in itchy skin diseases. Regulates antigen presentation on Langerhans cells; involved in drug-induced adverse reactions, atopic dermatitis and contact dermatitis. Downregulates NF-κB |
Cannabinoids | CB1, CB2 receptors | Nerves, keratinocytes, immune cells | Involved in antinociception (peripheral and central), anti-fibrotic; anti-inflammatory (contact dermatitis); regulates cell growth, differentiation, apoptosis. Involved in thermoregulation. |
Corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) | CRH receptors (CRHR1, CRH-R2) | CRH-R1: keratinocytes, mast cells CRH-R2: bone marrow mast cells | Induces release of histamine, cytokines, TNF-α, VEGF from mast cells. CRH-like immunoreactivity seen on sensory nerves (rat). |
Cytokines | Cytokine receptors (e.g., IL-2, IL-31) | Leukocytes, keratinocytes, endothelial cells, nerves | T cells release IL-31 during inflammation and activate monocytes and keratinocytes via the IL-31 receptor (IL-31R). IL-31R is upregulated in atopic dermatitis and prurigo. |
Endocannabinoids | Cannabinoid receptors (CB1, CB2) | Nerves, immune cells, keratinocytes, hair follicles | Antipruritic in the periphery. |
Endothelins (ETs) | Endothelin receptors (ETA, ETB) | Endothelium, mast cells | Induces burning itch. Degraded by chymase via ETA-receptor activation. |
TRP channels and agonists (heat, cold, acidosis, osmolar changes, capsaicin, menthol, camphor, eicosanoids, bradykinin, prostaglandins, various neurotrophins; aldehydes, formalin, nicotine, etc.) | Activation of transient receptor potential vanilloid 1 (TRPV1) sensitization of TRPV1 via activation of specific receptors (see in this table) | TRPV1 is expressed on sensory neurons, mast cells, epidermal and hair follicle keratinocytes, Langerhans cells, smooth muscle, and sebocytes TRPV2 expressed by sensory nerves and murine macrophages TRPV3 and TRPV4: expressed by sensory nerves and keratinocytes. TRPV6: keratinocytes. TRPA1: expressed by sensory nerves and keratinocytes | Short-term TRPV1 activation: induces pain and itch, depletes neuropeptides from sensory neurons. Long-term antipruritic effect of TRPV1 agonists (e.g., capsaicin): Suspend interplay between sensory neurons and mast cells. Affects epidermal and hair follicle proliferation, differentiation, apoptosis, and cytokine release. Increased expression in epidermal keratinocytes of prurigo nodularis patients. TRPV2: role in antigen presentation in mice. TRPV3 and TRPV4: role in sensory and keratinocyte function. TRPA1: role in pain, hypernociception, vasoregulation, barrier function. TRPV6: keratinocyte differentiation |
Gastrin-releasing peptide | Activates GRPR | GRP: sensory nerves. GRPR: superficial dorsal horn of spinal cord | GRP mediates itch but not pain (histamine dependent and independent). |
| Pruritogenic Stimuli (In Alphabetic Order) | Receptors | Sources, Receptors by which Expressed | Comments |
Histamine | Histamine receptors (H1R to H4R) | Sensory fibers | In humans, histamine induces itch by stimulating specific sensory fibers, whereas H1 (and to a lesser extent H2) antagonists reduce itch in numerous clinical trials. In mice, H3 antagonists induce scratching behavior, whereas H1 and H4 antagonists effectively suppress pruritus. |
Proteases, kallikreins, tryptase, trypsins, cathepsins, (MMP1) | Partly proteinase-activated receptors (PARs), tryptic enzymes | Keratinocytes, endothelial cells, mast cells, platelets | Massive itch behavior in mice overexpressing epidermal kallikrein-7. Potential role of other kallikreins. Chymase degrades pruritic and antipruritic peptides. Tryptase, trypsin and cathepsin S induce inflammation and itch by a neurogenic mechanism via PAR-2. Microbial proteases may induce itch and inflammation via PAR-2. PAR1 and PAR4 involved in pain. |
Kinins | Bradykinin receptors (B1R, B2R) | Endothelial cells, immunocytes | Bradykinin induces pain rather than pruritus. B2R antagonists reduce itch. |
Leukotriene B4 | Leukotriene receptors | Sensory nerves fibers, keratinocytes | Leukotriene B4 induces itch and is also involved in the substance P- and nociceptin-mediated induction of itch. |
Neurokinin A (NKA) and substance P (SP) | Tachykinin (neurokinin) receptors (NKRs) | Sensory nerve fibers | NKA: upregulates keratinocyte nerve growth factor expression. SP: at low (physiologically relevant) concentrations, primes mast cells. Mediates release of TNF-α, histamine, leukotriene B4, and prostaglandins from mast cells (agents involved in pruritus and burning). |
Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophins (NT-3, NT-4) | Specific receptors | Keratinocytes, mast cells, fibroblasts, eosinophils | NGF levels enhanced in atopic dermatitis. Induces tryptase release from mast cells. Inducible by histamine. |
TrkA: NGF | TrkA: enhanced in keratinocytes during inflammation. | ||
TrkB: NT-4, BDNF | NT-4: enhanced in atopic dermatitis and induces sprouting of sensory nerves. | ||
TrkC: NT-3 | BDNF: increases eosinophil chemotaxis levels in atopic dermatitis and inhibits apoptosis. Neurotrophins sensitize receptive nerve endings and upregulate neuronal neuropeptides and TRPV1. | ||
Opioids | μ, κ, δ opioid receptors (partly receptor-independent cell activation) | Nerves, keratinocytes | Antipruritic effect of μ-opioid antagonists (central effect) and κ-opioid agonists (spinal cord level). Opioid agonists do not provoke itch on injection or intradermal application. μ-Opioid receptor upregulated in atopic dermatitis. |
Pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) | VPAC receptors | Autonomic and sensory nerve fibers, lymphocytes, dermal endothelial cells, Merkel cells | PACAP: involved in flush, vasodilation, pain, neurodegeneration. Induces release of histamine from mast cells. VIP: Histamine release from mast cells, allodynia (no allodynia in atopic dermatitis) intensifies ACh-induced itch in atopic dermatitis patients (together with ACh). |
Prostaglandins | Prostanoid (P) receptors | Sensory nerve fibers, keratinocytes | Prostaglandin E2 induces itch sensitization in humans but not in mice. Prostaglandin D2 reduces immunoglobulin E-mediated scratching in mice. Thromboxane A2 induces itch in mice. |
ATP = adenosine triphosphate; IL = interleukin; TNF-α = tumor necrosis factor-α; VEGF = vascular endothelial growth factor. | |||
Neuromediator | Receptor | Source | Target Cells/Function |
|---|---|---|---|
Acetylcholine | Nicotinergic and muscarinergic acetylcholine receptors | Autonomic cholinergic nerves, keratinocytes, lymphocytes, melanocytes | Innervation of sweat glands and arteriovenous anastomoses; keratinocyte and lymphocyte differentiation, proliferation, adhesion, migration. α7-Nicotinergic receptor modulates keratinocyte function, modulates skin microcirculation; involved in atopic dermatitis. |
Adenosine, ATP | Purinergic receptors | Nerves, keratinocytes, endothelial cells, immune cells | Involved in pain and hypernociception; neuronal cell death; involved in vasoregulation and immune response (cytokine release, cell adhesion molecules). |
Catecholamine, noradrenaline | Adrenergic receptors | Autonomic adrenergic nerves, keratinocytes, melanocytes | Innervation of blood vessels, erector pili muscles; pain transmission; regulation of activity in natural killer cells and monocytes; induction of apoptosis in lymphocytes. Regulate dendritic cell function. β-Adrenergic-induced inhibition of keratinocyte migration. |
CGRP | Involved in sweating. | ||
Galanin and galanin-like peptides | Galanin receptors (GPCRs) | Sensory nerves | Inhibit inflammatory edema by reduction of microvascular blood flow via Gal3R. |
Substance P | Tachykinin (neurokinin) receptor | Sensory nerve fibers, keratinocytes (inducible) | Mediation of skin edema, pruritus; upregulation of cell adhesion molecule expression on keratinocytes and endothelial cells; induction of release of IL-8, TNF-α, histamine, tryptase, leukotriene B4, prostaglandin D2; regulation of sebaceous glands; involved in inflammatory pain, contact dermatitis, immunomodulation, tumorigenesis and metastasis. Released by proteinase-activated receptor 2 (PAR-2) agonists. |
Neurokinin A | Tachykinin (neurokinin) receptor | Sensory nerve fibers | Upregulation of keratinocyte nerve growth factor expression. |
Vasoactive intestinal peptide | VPAC receptors | Sensory nerve fibers, Merkel cells | Sweat secretion, vasodilation; proliferation, migration of keratinocytes; histamine release from mast cells. Downregulation of VPAC2 receptor in mast cells in atopic dermatitis. |
Pituitary adenylate cyclase-activating polypeptide | VPAC receptors | Autonomic and sensory nerve fibers, lymphocytes, dermal endothelial cells | Vasodilatation, immunomodulation; effect on T cells and macrophages; modulation of mast cell function; inhibition of antigen-induced apoptosis of mature T lymphocytes; downregulation of proinflammatory cytokines and chemokines in T cells; upregulation of cytokines and cell adhesion molecules in dermal microvascular endothelial cells; nociception. |
Calcitonin gene-related peptide (CGRP) | CGRP receptors | Sensory nerve fibers | Keratinocyte and endothelial cell proliferation; stimulation of cytokine production. Increased CGRP nerves in atopic dermatitis and nummular eczema; released by PAR-2 stimulation. |
Pro-opiomelanocortin (POMC) | Melanocortin receptors | Melanocytes, keratinocytes, endothelial cells, Langerhans cells, mast cells, fibroblasts, monocytes, macrophages | Antagonism of effects of proinflammatory cytokines (IL-17α, IL-1β, IL-6, TNF-α, endotoxins); upregulation of IL-10; induction of release of histamine from mast cells; inhibition of nuclear factor κ B. Thioredoxin regulates POMC genes. MSH-induced exocytosis from melanosomes. Melanocortin 1 receptor expressed on human keratinocytes. Antioxidative and cytoprotective. |
From this background, it is clear that a better cellular and molecular understanding of the complex skin–nervous system interactions opens an avenue for future therapeutic approaches to skin disease.
Taken as a whole, present information clearly indicates a crucial role for the neuronal skin network in influencing a variety of physiologic and pathophysiologic functions such as host defense, inflammation, pruritus, pain, burning, wound healing, and probably cancer (e.g., by modulating angiogenesis).
The CNS proper is connected to skin either directly via efferent nerves or CNS-derived mediators, or indirectly via the adrenal glands or immune cells.1 Cutaneous nerves also respond to internal stimuli from the circulation (e.g., pH changes, osmotic changes, bradykinin, cytokines) or the skin itself and to emotions (internal trigger factors).2 Under normal circumstances, the sensory and the autonomic nervous systems modulate important biologic functions such as body temperature, blood flow, and cell growth. Mechanically induced nerve impulses transmit information such as pressure to the CNS. Chemical- or heat-responsive afferent nerve fibers are involved in recognizing dangerous signals. Thus, normally the innervated skin is a crucial barrier in protecting the body from danger from the external environment. This is also supported by the finding that not only the dermis but also the epidermis is highly innervated.4 Interactions between the central and peripheral nervous system have been implicated to play a role in thermoregulation,1,5 the pathophysiology of various skin diseases such as psoriasis,6–8 atopic dermatitis,9 acne,10 wound healing,11 as well as hair loss and regrowth.12–14
Our knowledge about the involvement of the spinal cord in regulating specific chronic pain sensations in human skin, its role in modulating pruritus, or modulating inflammatory stimuli transduced from the periphery to the brain is very limited. We know that μ-opioids such as morphine can induce pruritus while being analgesic when injected intrathecally. In contrast, κ-opioids exert antipruritic effects, probably by the inhibition of the μ-opioid receptor. These results strongly indicate a role of opioids in the regulation of pain and pruritus on the spinal cord level. Moreover, gastrin-releasing peptide (bombesin) released to the central nerve endings in the spinal cord activates the gastrin-releasing peptide receptor (GRPR) on postsynaptic spinal neurons, thereby regulating selectively itch transduction but not pain.15,16 Together, these data implicate the spinal cord as an important regulator of skin–nervous system interactions as observed during inflammation, pruritic diseases, and pain, and may be a target for future therapies.
Anatomy and Physiology of the Skin Nervous System
Most nerve fibers are found in the middermis and the papillary dermis. Region-specific differences can be observed with respect to the mucocutaneous border, glabrous skin, and hairy skin.17 In the epidermis, sensory nerves are linked to keratinocytes, melanocytes, Langerhans cells (LC), and Merkel cells. Cutaneous nerve fibers are principally sensory, with an additional complement of autonomic nerve fibers.18,19 In contrast to sensory nerves, autonomic nerves never innervate the epidermis in mammals. Sensory nerves innervate the epidermis and dermis as well as the subcutaneous fatty tissue20–22 (see eFig. 102-0.1).
Sensory nerves are categorized into two groups: (1) the epidermal and (2) the dermal skin nerve organs. The epidermal skin nerve organs consist of free nerve endings or nerve organs (e.g., Merkel cells). In the dermis, there are free sensory nerve endings, the hair nervous network (Pinkus discs), and the encapsulated endings [Ruffini, Meissner, Krause, and Vater–Pacini (vibration) corpuscles, and mucocutaneous end organ]. These can be subdivided into four groups: (1) A-α fibers (12–22 nm) are highly myelinated, show a fast conduction velocity (70–120 m/second), and are associated with muscular spindles and tendon organs. (2) A-β fibers are moderately myelinated (6–12 μm) and innervate touch receptors. (3) A-δ fibers have a thin myelin sheath (1–5 μm), show an intermediate conduction velocity (4–30 m/second), and are generally polymodal. (4) The slow-conducting C fibers (0.5–2.0 m/second) are unmyelinated and thin (0.2–1.5 μm) (nociceptors). A-β and A-δ fibers are mostly mechanically sensitive afferents (type I) localized on hairy and glabrous skin and show a long latency to heat. A subpopulation of A-δ fibers on hairy skin are mechanically insensitive (type II). A-δ fibers constitute approximately 80% of primary sensory nerves sprouting from DRG, whereas C fibers make up approximately 20% of the primary afferents.23,24
C-fibers are either polymodal nociceptors, which can respond to chemical (c+), temperature changes (h+) or mechanical (m+) stimuli, or more specialized, which only respond to a combination (C-c+h+m−) or a single stimulus (C-c−h−m+). Among human peripheral nerves, 45% of the cutaneous afferent nerves belong to a subtype of sensory nerves that are both mechano- and heat-responsive C-fibers (C-c−m+h+).25 However, 13% of these nerves are only mechanosensitive (C-m+), 6% only heat sensitive (C-h+), 24% are neither heat nor mechanoresponsive (C-m−h−), and approximately 12% are of sympathetic (cholinergic) origin. Fifty-eight percent of C-m+h+ respond to mustard oil, whereas 30% of C-m+ or (C-m−h−) do so indicating the existence of chemosensitive fibers among the other subtypes.25
Sensory nerves percept cutaneous stimuli such as warmth, cold, or touch. The nerves for warmth are predominantly unmyelinated C-fibers, a subpopulation of A-δ fibers respond to gentle cooling, whereas selective C-fibers become activated during noxious cold. Many subtypes of cells respond to touch and play an important role in mechanically induced pain. Thus, our body system has designed less selective as well as highly specialized nociceptors in order to guarantee body integrity and survival.26
A specific receptor distribution on these different sensory nerve subtypes appears to be important for the various functions (temperature, chemical, mechanical) and the sensations, which may derive thereof (prickling, stinging, burning, pain, itch). For example, mechanoreceptors exclusively express the T-type calcium channel Ca(v)3.2 in the dorsal root ganglion of D-hair receptors. The sodium channels Nav1.8 (SNS/PN3) and Nav1.9 (SNS/SNS2) are expressed by both peptidergic as well as nonpeptidergic IB4+ (isolectin B4 from Griffonia simplicifolia) neurons and have been shown to be critically involved in certain subtypes of pain.27 Only certain small-diameter primary afferents express the transient receptor potential vanilloid-1 (TRPV1) receptor which is critically involved in heat sensation.28 Only nonpeptidergic (poor peptidergic) sensory fibers express the purinergic P2×3 receptor. Exogenous factors like trauma, UV-radiation, temperature changes, microbial agents, toxins, or allergens, as well as endogenous inflammatory triggers such as pH changes or stress hormone responses are able to stimulate the activation and/or sensitization of certain sensory nerves. The cellular events that transmit a stimulus (e.g., UV radiation) to a certain response (burning pain) via activation of a certain pathway (activation of pain fibers but not itch fibers and vice versa) are only poorly understood.29,30
Compared to sensory nerves, autonomic nerves represent only a minority of cutaneous nerve fibers. In human skin, autonomic nerve fibers are derived almost completely from sympathetic (cholinergic) and rarely from parasympathetic (also cholinergic) neurons.31 The distribution of autonomic nerves is restricted to the dermis, where they innervate blood vessels, arteriovenous anastomoses, lymphatic vessels, erector pili muscles, eccrine glands, apocrine glands, and hair follicles.32
Postganglionic autonomic nerves in the skin predominantly generate acetylcholine, although observations have revealed an additional role for neuropeptides. For example, neuropeptide Y (NPY) and atrial natriuretic peptide33 are expressed solely by autonomic nerve fibers.34
The cutaneous autonomic nervous system adjusts sweat gland function, thereby regulating body temperature, and modulates water and electrolyte balance in various organs. Under pathophysiologic conditions, autonomic nerves are involved in hyperhidrosis or hypohidrosis, congenital sensory neuropathy type IV, progressive segmental hypohidrosis, diabetic neuropathy, syringomyelia, lepra, and dysfunction after sympathectomy.35–38
Autonomic nerves exert their effects mainly by releasing classical neurotransmitters (noradrenaline, acetylcholine) or—to a lesser extent—certain neuropeptides like vasoactive intestinal peptide (VIP). In contrast, primary afferent (sensory) nerves release different classes of molecules such as neuropeptides, prostanoids, or nitric oxide (NO).1
Autonomic nerve fibers are crucially involved in the regulation of vascular effects in the skin. Sympathetic nerve fibers release noradrenalin and/or NPY to innervate arterioles, arteriovenous anastomoses, and venous sinusoids, which results in vasoconstriction, whereas parasympathetic nerves mediate vasodilation through activation of venous sinusoids by the release of acetylcholine and vasoactive intestinal peptide (VIP)/peptide histidine methionine39–42 (eTable 102-0.1 and Table 102-1). Of note, C-fiber nociceptors can develop responsiveness to adrenergic neurotransmitters by upregulating the corresponding receptors during trauma or inflammation. Thus, the sensory and the autonomic nervous systems communicate and interact in disease on the molecular level.
Small arteries, arterioles, and the arteriovenous anastomoses are richly supplied with noradrenergic nerves.43 Previous studies suggested that this system is cholinergic and involves a cotransmitter, possibly VIP.44 Cholinergic sympathetic nerves are also known to stimulate eccrine sweat glands via muscarinic receptors,31 whereas higher concentrations of acetylcholine induce an axon-reflex flare mediated via nicotinic receptors. In the vasoconstrictor system, the transmitter appears to be norepinephrine along with one or more cotransmitters. The best-characterized sympathetic cotransmitters that participate in the regulation of blood flow include adenosine triphosphate45 and NPY.46
Interestingly, even without intact sensory or autonomic function the skin reveals a nonneurogenic vasodilator and nonneurogenic vasoconstriction response. The mechanisms for the nonneurogenic vasodilator and vasoconstrictor components of the response to direct cooling are poorly understood but may involve several pathways including cholinergic stimulation, NO and neuropeptides.5,47
Biochemistry and Cell Biology of the Cutaneous Nervous System
As outlined above, many subtypes of nerve fibers exist to cover the multiple functions of the skin nervous system to maintain or reestablish body integrity. This chapter focuses on the role of neuropeptides, TRP channels, and cytokines in the skin (Table 102-1).
Classically, neuropeptides range from as few as 4 to more than 40 amino acids. Because they are released by nerve endings and modulate various biologic functions, they were originally defined as neuropeptides. Later, these molecules were also found to be generated by nonneuronal cells (e.g., epithelial cells and immune cells). Therefore, the designation regulatory peptide may be more appropriate.48 These peptides mainly activate members of the G protein-coupled receptor superfamily with seven transmembrane domains. To date more than 20 neuropeptides, including SP, neurokinin A (NKA), neurotensin, CGRP, VIP, pituitary adenylate cyclase-activating polypeptide (PACAP), peptide histidine-isoleucinamide, NPY, somatostatin (SST), dynorphin, β-endorphin, enkephalin, galanin, secretoneurin, melanocyte-stimulating hormone (MSH), thyroid-stimulating hormone (TSH), or corticotropin-releasing hormone (CRH), have been detected in the skin.1,2,49 Table 102-1 lists the neuropeptides, ion channels, and other molecules like NO involved in pruritus, pain, and inflammation. eFigure 102-0.1 and Fig. 102-1 depict the basic concepts and knowledge concerning these neuropeptides.
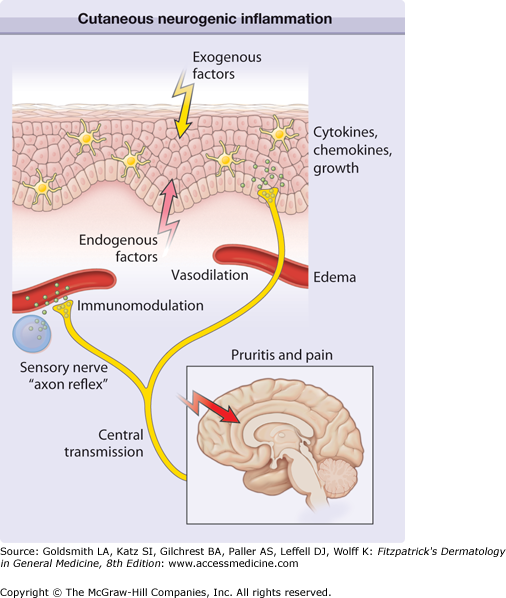
Figure 102-1
Cutaneous neurogenic inflammation. Exogenous trigger factors (heat, scratching, irritants, allergens, ultraviolet light, microbiologic agents) or endogenous trigger factors (pH changes, cytokines, kinins, histamine, proteases, neurotransmitters, hormones, stress) may directly or indirectly stimulate nerve endings from primary afferent neurons. Signals are transmitted to the central nervous system and thereby affect regions involved in pruritus, pain, somatosensory reactions (scratching), and probably emotional responses. In addition, peripheral nerve endings stimulate neighboring afferent nerve fibers in the dermis and epidermis in a process known as axon reflex. Stimulated release of neuropeptides results in vascular responses (triple response of Lewis, erythema by vasodilation, and edema by plasma extravasation), modulation of immunocyte function (e.g., mediator release from mast cells), and regulation of mediator release (cytokines, chemokines, growth factors) from keratinocytes and Langerhans cells.
Various neuropeptides are produced and released by a subpopulation of unmyelinated afferent neurons (C fibers) known as C-polymodal nociceptors. In addition, it has been shown that cutaneous cells themselves, such as keratinocytes, microvascular endothelial cells, Merkel cells, fibroblasts, and leukocytes, are capable of releasing regulatory peptides under physiologic or pathophysiologic circumstances.
In the skin, nerves are necessarily closely linked to the vascular system. Dermal blood vessels are tightly associated with sensory and autonomic nerve fibers, they also synthesize neuropeptides and they express receptors for neuropeptides. Arterial sections of arteriovenous anastomoses, precapillary sphincters of metarterioles, arteries, and capillaries appear to be the most intensely innervated regions. Large increases in skin blood flow provide the necessary augmentation of convective heat loss during environmental heat exposure and/or exercise, and the reflex cutaneous vasoconstriction is key to preventing excessive heat dissipation during cold exposure. Sensory nerves are important for vasodilation and neuropeptides from sympathetic neurons such as NPY mediate vasoconstriction, which supports an important role for neuropeptides in thermoregulation, blood flow during inflammation or tumorigenesis, as well as activation of endothelial cells and smooth muscle cells. Both endothelial cells and smooth muscle cells respond to neuronal modulation in inflammatory diseases such as atopic dermatitis and rosacea and during host defense, neovascularization, and wound healing. Sensory as well as autonomic nerves modulate the function of sweat glands, sebaceous glands, apocrine glands, and the pilosebaceous unit. In the following, we emphasize the biological role of certain neuromediators and neurohormones in more detail.
![]() In the skin, tachykinin-immunoreactive sensory nerves are often associated with dermal blood vessels, mast cells, hair follicles, or epidermal cells (Fig. 102-1).50 Increased epidermal substance P (SP)-immunoreactive nerve fibers have been observed in certain inflammatory human skin diseases such as psoriasis, atopic dermatitis, and contact dermatitis.51,52 Moreover, several immune cells are capable of generating SP induced by stress, inflammation, or infection.53–55 For example, SP appears to be involved in keratinocyte/antigen-presenting cell interactions during chronic stress,54 T cell regulation,56 natural killer cell activation,57 innate host defense,58 HIV-associated psoriasis,53,59 wound healing,60 murine hair follicle apoptosis,61 genital herpes infection,62 and immunosurveillance during experimentally induced tumor growth (murine melanoma).63 SP may be also involved in inflammation and host responses of the CNS64 as well as transmitting sensory signals (neurogenic inflammation, pain, pruritus) to the CNS48,65. In addition, in a murine disease model, the NK1 receptor was recently shown to play an important role in the development of airway inflammation and hyperresponsiveness.66
In the skin, tachykinin-immunoreactive sensory nerves are often associated with dermal blood vessels, mast cells, hair follicles, or epidermal cells (Fig. 102-1).50 Increased epidermal substance P (SP)-immunoreactive nerve fibers have been observed in certain inflammatory human skin diseases such as psoriasis, atopic dermatitis, and contact dermatitis.51,52 Moreover, several immune cells are capable of generating SP induced by stress, inflammation, or infection.53–55 For example, SP appears to be involved in keratinocyte/antigen-presenting cell interactions during chronic stress,54 T cell regulation,56 natural killer cell activation,57 innate host defense,58 HIV-associated psoriasis,53,59 wound healing,60 murine hair follicle apoptosis,61 genital herpes infection,62 and immunosurveillance during experimentally induced tumor growth (murine melanoma).63 SP may be also involved in inflammation and host responses of the CNS64 as well as transmitting sensory signals (neurogenic inflammation, pain, pruritus) to the CNS48,65. In addition, in a murine disease model, the NK1 receptor was recently shown to play an important role in the development of airway inflammation and hyperresponsiveness.66
![]() During wound healing, SP may promote the healing process by affecting the expression of both epidermal growth factor and epidermal growth factor receptor in the granulation tissues as demonstrated in a rat model.67 In addition, keratinocyte nerve growth factor (NGF) is induced by sensory nerve-derived neuropeptides such as SP and neurokinin A (NKA).68 Finally, SP may modulate cutaneous inflammatory responses by upregulation of cell adhesion molecule expression on keratinocytes.69
During wound healing, SP may promote the healing process by affecting the expression of both epidermal growth factor and epidermal growth factor receptor in the granulation tissues as demonstrated in a rat model.67 In addition, keratinocyte nerve growth factor (NGF) is induced by sensory nerve-derived neuropeptides such as SP and neurokinin A (NKA).68 Finally, SP may modulate cutaneous inflammatory responses by upregulation of cell adhesion molecule expression on keratinocytes.69
![]() In cultured normal human fibroblasts, a moderate amount of preprotachykinin-A was found, which was significantly upregulated by exogenous SP. Accordingly, SP was found to promote human fibroblast chemotaxis in a dose-dependent manner.70 The effects of SP on both fibroblast proliferation and transforming growth factor-β1 (TGF-β1) mRNA expression could be antagonized by a selective NK1R antagonist, suggesting that SP may play an important role in phenotype changes of fibroblast proliferation. In cultured rheumatoid fibroblast-like synoviocytes, SP enhances cytokine-induced VCAM-1 expression in a dose-dependent manner, probably via NK1R activation.
In cultured normal human fibroblasts, a moderate amount of preprotachykinin-A was found, which was significantly upregulated by exogenous SP. Accordingly, SP was found to promote human fibroblast chemotaxis in a dose-dependent manner.70 The effects of SP on both fibroblast proliferation and transforming growth factor-β1 (TGF-β1) mRNA expression could be antagonized by a selective NK1R antagonist, suggesting that SP may play an important role in phenotype changes of fibroblast proliferation. In cultured rheumatoid fibroblast-like synoviocytes, SP enhances cytokine-induced VCAM-1 expression in a dose-dependent manner, probably via NK1R activation.
![]() Previous studies using the tachykinin NK1R antagonist, SR140333, have indicated that cutaneous edema can be mediated by NK1Rs and is independent of histamine effects.71 This finding is supported by experiments using NK1R knockout mice, which showed that intradermally injected SP, NK1R agonists (GR-73632), and the mast cell-degranulating agent, compound 48/80, induced dose-dependent cutaneous edema in wild-type mice and this did not occur in knockout mice.72 Despite of its disappointing role in pain, the NK1R antagonist aprepitant (Emend®) has been shown to play an important role to block chemotherapy-induced emesis, as well as pruritus in patients with Sezary-syndrome, solid tumors, or chronic pruritus.73–75
Previous studies using the tachykinin NK1R antagonist, SR140333, have indicated that cutaneous edema can be mediated by NK1Rs and is independent of histamine effects.71 This finding is supported by experiments using NK1R knockout mice, which showed that intradermally injected SP, NK1R agonists (GR-73632), and the mast cell-degranulating agent, compound 48/80, induced dose-dependent cutaneous edema in wild-type mice and this did not occur in knockout mice.72 Despite of its disappointing role in pain, the NK1R antagonist aprepitant (Emend®) has been shown to play an important role to block chemotherapy-induced emesis, as well as pruritus in patients with Sezary-syndrome, solid tumors, or chronic pruritus.73–75
![]() In the skin, VIP-like immunoreactivity was detected in nerve fibers associated with dermal vessels, glands such as sweat, apocrine, and Meibomian glands, hair follicles, and Merkel cells. VIP immunoreactivity was less abundant than SP immunoreactivity in the epidermal layer.76,77 VIP-staining fibers can be also found in close anatomical connection to mast cells.78–80,1,29,52 In the skin, VIP regulates temperature homeostasis and sweat production.81–85 VIP is also involved in neurogenic inflammation possibly through histamine release from mast cells, inducing bradykinin-mediated edema86,87 as well as stimulating cytokine release and growth factor release from keratinocytes.88 VIP may also play a role during infection. For example, antibodies against VIP were found in patients with HIV and were more prevalent in asymptomatic carriers and their titer correlated with disease progression.89
In the skin, VIP-like immunoreactivity was detected in nerve fibers associated with dermal vessels, glands such as sweat, apocrine, and Meibomian glands, hair follicles, and Merkel cells. VIP immunoreactivity was less abundant than SP immunoreactivity in the epidermal layer.76,77 VIP-staining fibers can be also found in close anatomical connection to mast cells.78–80,1,29,52 In the skin, VIP regulates temperature homeostasis and sweat production.81–85 VIP is also involved in neurogenic inflammation possibly through histamine release from mast cells, inducing bradykinin-mediated edema86,87 as well as stimulating cytokine release and growth factor release from keratinocytes.88 VIP may also play a role during infection. For example, antibodies against VIP were found in patients with HIV and were more prevalent in asymptomatic carriers and their titer correlated with disease progression.89
![]() VIP is involved in neuro-immunomodulation.90 This cytokine-like peptide exerts a broad spectrum of anti-inflammatory effects in mammals including humans.91 In murine T cells, VIP has the capacity to modulate CD4+CD25+ Foxp3-expressing regulatory T cells in vivo. Application of VIP into T cell receptor transgenic mice resulted in the expansion of T cells that inhibited the responder T cell proliferation, increased the level of CD4+CD25+ Treg cells, inhibited delayed-type hypersensitivity in T cell receptor-transgenic (TCR-Tg) hosts, and prevented graft-versus-host disease in vivo.92
VIP is involved in neuro-immunomodulation.90 This cytokine-like peptide exerts a broad spectrum of anti-inflammatory effects in mammals including humans.91 In murine T cells, VIP has the capacity to modulate CD4+CD25+ Foxp3-expressing regulatory T cells in vivo. Application of VIP into T cell receptor transgenic mice resulted in the expansion of T cells that inhibited the responder T cell proliferation, increased the level of CD4+CD25+ Treg cells, inhibited delayed-type hypersensitivity in T cell receptor-transgenic (TCR-Tg) hosts, and prevented graft-versus-host disease in vivo.92
![]() In macrophages, VIP and PACAP protect mice from lethal endotoxemia through the inhibition of TNF-α and interleukin-6 (IL-6) suggesting a protective role of both neuropeptides in innate immunity93 by downregulating TNF-α production.94 VIP is also involved in modulating innate immunity by downregulating toll-like receptor 4 (TLR4) expression and TLR4-mediated chemokine generation (CCL2, CXCL8).95
In macrophages, VIP and PACAP protect mice from lethal endotoxemia through the inhibition of TNF-α and interleukin-6 (IL-6) suggesting a protective role of both neuropeptides in innate immunity93 by downregulating TNF-α production.94 VIP is also involved in modulating innate immunity by downregulating toll-like receptor 4 (TLR4) expression and TLR4-mediated chemokine generation (CCL2, CXCL8).95
![]() PACAP is present in sensory and autonomic nerve fibers of DRG, the spinal cord and the adrenal glands suggesting involvement in sensory and nociceptive pathways.96,97 Moreover, PACAP-immunoreactive fibers are sensitive to capsaicin.97 In various organs of rodents and humans, PACAP displays neuroprotective, regenerative, and immunomodulatory functions. In severe combined immunodeficiency (SCID) mice, CD4+ T cells appear to induce PACAP gene expression, suggesting a regulatory role of immune cells on PACAP-induced immunomodulation and nerve regeneration.98
PACAP is present in sensory and autonomic nerve fibers of DRG, the spinal cord and the adrenal glands suggesting involvement in sensory and nociceptive pathways.96,97 Moreover, PACAP-immunoreactive fibers are sensitive to capsaicin.97 In various organs of rodents and humans, PACAP displays neuroprotective, regenerative, and immunomodulatory functions. In severe combined immunodeficiency (SCID) mice, CD4+ T cells appear to induce PACAP gene expression, suggesting a regulatory role of immune cells on PACAP-induced immunomodulation and nerve regeneration.98
![]() In the skin, PACAP was detected in sensory nerve fibers99,100 coexisting with VIP, SP or CGRP, respectively, all of which may play an important role in inflammatory skin diseases like psoriasis, urticaria, or atopic dermatitis,101 The distribution of PACAP-3899,100 and the presence of the high-affinity PACAP1-receptor (PAC1R)99 was described in normal and inflamed human skin. The concentration of PACAP-38 appears to be enhanced in lesional skin of psoriasis patients.99 Moreover, the peptide level was significantly lower in nonlesional psoriatic skin than in lesional psoriatic skin, but was about twice as much as in normal human skin. Interestingly, immunoreactivity was significantly increased at the dermal–epidermal border in psoriasis99 around hair follicles and close to sweat glands of normal skin. In contrast, no significant increase of positive nerve fibers was observed around blood vessels. PACAP appears to be involved in cutaneous inflammation, for example, by releasing histamine from mast cells.100 PACAP modulates the function of blood vessels,102–106 T cells,90 or macrophages.197 PACAP appears to be an important vasoregulator in human skin in vivo.84,107
In the skin, PACAP was detected in sensory nerve fibers99,100 coexisting with VIP, SP or CGRP, respectively, all of which may play an important role in inflammatory skin diseases like psoriasis, urticaria, or atopic dermatitis,101 The distribution of PACAP-3899,100 and the presence of the high-affinity PACAP1-receptor (PAC1R)99 was described in normal and inflamed human skin. The concentration of PACAP-38 appears to be enhanced in lesional skin of psoriasis patients.99 Moreover, the peptide level was significantly lower in nonlesional psoriatic skin than in lesional psoriatic skin, but was about twice as much as in normal human skin. Interestingly, immunoreactivity was significantly increased at the dermal–epidermal border in psoriasis99 around hair follicles and close to sweat glands of normal skin. In contrast, no significant increase of positive nerve fibers was observed around blood vessels. PACAP appears to be involved in cutaneous inflammation, for example, by releasing histamine from mast cells.100 PACAP modulates the function of blood vessels,102–106 T cells,90 or macrophages.197 PACAP appears to be an important vasoregulator in human skin in vivo.84,107
![]() CGRP is one of the most prominent neuropeptides in the skin and often colocalized with either SP or SST (Fig. 102-1).108,109 CGRP-immunoreactive nerves are often associated with mast cells,110 Merkel cells,111 melanocytes,112 keratinocytes, and LC113,114 which are stimulated under inflammatory conditions.115 In LC, CGRP inhibits antigen-presentation capacities of the immune cells. CGRP-immunoreactivity was detected in association with smooth muscle cells and blood vessels.116,117 CGRP has also potent vasoactive effects,118 and can increase keratinocyte proliferation112 or cytokine release. CGRP and SP also upregulated IL-8R mRNA expression but not IL-8 production in HaCaT cells.119 CGRP also mediates anti-inflammatory and neurotrophic effects.120 CGRP modulates macrophages,121 neutrophils,122 and dermal microvascular endothelial cells.109,123 CGRP is one of the most potent vasodilatatory mediators on small and large vessels124–127 and potentiates microvascular permeability and edema formation caused by SP or NKA. CGRP and endothelin are colocalized in endothelial cells suggesting autoregulatory mechanisms for blood flow.128
CGRP is one of the most prominent neuropeptides in the skin and often colocalized with either SP or SST (Fig. 102-1).108,109 CGRP-immunoreactive nerves are often associated with mast cells,110 Merkel cells,111 melanocytes,112 keratinocytes, and LC113,114 which are stimulated under inflammatory conditions.115 In LC, CGRP inhibits antigen-presentation capacities of the immune cells. CGRP-immunoreactivity was detected in association with smooth muscle cells and blood vessels.116,117 CGRP has also potent vasoactive effects,118 and can increase keratinocyte proliferation112 or cytokine release. CGRP and SP also upregulated IL-8R mRNA expression but not IL-8 production in HaCaT cells.119 CGRP also mediates anti-inflammatory and neurotrophic effects.120 CGRP modulates macrophages,121 neutrophils,122 and dermal microvascular endothelial cells.109,123 CGRP is one of the most potent vasodilatatory mediators on small and large vessels124–127 and potentiates microvascular permeability and edema formation caused by SP or NKA. CGRP and endothelin are colocalized in endothelial cells suggesting autoregulatory mechanisms for blood flow.128
![]() CGRP may be regulated by UV-mediated responses because irradiation of the skin decreased CGRP mRNA expression in DRG.129 CGRP also induces melanocyte proliferation by upregulating melanogenesis and enhances melanocyte dendricity by inducing keratinocyte-derived melanotropic factors130 indicating a modulatory role of CGRP for epidermal melanocyte function. Therapeutically, CGRP receptor antagonists like olcegepant or telcagepant have been proven to be effective for the treatment of migraine indicating a role of this receptor in neurogenic diseases and neurogenic inflammation.131,132
CGRP may be regulated by UV-mediated responses because irradiation of the skin decreased CGRP mRNA expression in DRG.129 CGRP also induces melanocyte proliferation by upregulating melanogenesis and enhances melanocyte dendricity by inducing keratinocyte-derived melanotropic factors130 indicating a modulatory role of CGRP for epidermal melanocyte function. Therapeutically, CGRP receptor antagonists like olcegepant or telcagepant have been proven to be effective for the treatment of migraine indicating a role of this receptor in neurogenic diseases and neurogenic inflammation.131,132
![]() SST is described as an inhibitor of exocrine and endocrine secretion from a variety of tissues, and a predominantly antiproliferative molecule with tumor-suppressive properties that are mediated by tyrosine phosphatases.133,134 It also inhibits proliferation of T cells135 and leukocyte recruitment during the initial phase of inflammation.136 SST is released by sensory nerves which may have an immunosuppressive role in some basophil-dependent hypersensitivity reactions.137 However, SST may also stimulate histamine release from human skin mast cells138 and may be involved in the pathophysiology of atopic dermatitis and mastocytosis.139–141
SST is described as an inhibitor of exocrine and endocrine secretion from a variety of tissues, and a predominantly antiproliferative molecule with tumor-suppressive properties that are mediated by tyrosine phosphatases.133,134 It also inhibits proliferation of T cells135 and leukocyte recruitment during the initial phase of inflammation.136 SST is released by sensory nerves which may have an immunosuppressive role in some basophil-dependent hypersensitivity reactions.137 However, SST may also stimulate histamine release from human skin mast cells138 and may be involved in the pathophysiology of atopic dermatitis and mastocytosis.139–141
![]() Somatostatin receptors were detected in inflammatory lesions of patients suffering from rheumatoid arthritis, sarcoidosis, and granulomatosis with polyangiitis (Wegener’s).142,143 Treatment with octreotide of two patients with sarcoid granulomas143 or systemic sclerosis144 resulted in clinical improvement suggesting a role of SST analogs in the treatment of granulomatous diseases.
Somatostatin receptors were detected in inflammatory lesions of patients suffering from rheumatoid arthritis, sarcoidosis, and granulomatosis with polyangiitis (Wegener’s).142,143 Treatment with octreotide of two patients with sarcoid granulomas143 or systemic sclerosis144 resulted in clinical improvement suggesting a role of SST analogs in the treatment of granulomatous diseases.
![]() Opioids are peptidergic neurotransmitters with antinociceptive capacities and are divided into three classes: (1) endorphins, (2) enkephalins, and (3) dynorphins. Opioid receptors (OR) are classified into three subtypes: (1) μ-, (2) κ- and (3) δ- (Table 102-1).
Opioids are peptidergic neurotransmitters with antinociceptive capacities and are divided into three classes: (1) endorphins, (2) enkephalins, and (3) dynorphins. Opioid receptors (OR) are classified into three subtypes: (1) μ-, (2) κ- and (3) δ- (Table 102-1).
![]() In the skin, POMC peptides are expressed by melanocytes, keratinocytes, adnexal epithelial cells, sebocytes, microvascular endothelial cells, LCs, mast cells, and fibroblasts as well as by immune cells such as monocytes, dendritic cells, and macrophages.145 The skin and in particular the hair follicle seems to generate the whole armada of mediators generated by the hypothalamic-pituitary-adrenal (HPA) axis such as β-endorphin, MSH, ACTH, CRH, their receptors [μ-opioid receptor (μOR), MC-R, ACTH-R, CRH-R], and enzymes regulating POMC peptide function like PC1, and PC2.146
In the skin, POMC peptides are expressed by melanocytes, keratinocytes, adnexal epithelial cells, sebocytes, microvascular endothelial cells, LCs, mast cells, and fibroblasts as well as by immune cells such as monocytes, dendritic cells, and macrophages.145 The skin and in particular the hair follicle seems to generate the whole armada of mediators generated by the hypothalamic-pituitary-adrenal (HPA) axis such as β-endorphin, MSH, ACTH, CRH, their receptors [μ-opioid receptor (μOR), MC-R, ACTH-R, CRH-R], and enzymes regulating POMC peptide function like PC1, and PC2.146
![]() β-Endorphin can be generated in human keratinocytes via activation of the μOR by ultraviolet radiation. In human melanocytes, β-endorphin has melanogenic, mitogenic, and dendritogenic effects in vitro, and expresses μOR.147 Accordingly, human hair follicle melanocytes also express β-endorphin and μOR, especially in glycoprotein-100-positive cells, suggesting a role of hair growth in an autocrine fashion. Thus, β-endorphin may be involved in pigmentation and hair growth. Recent findings indicate that β-endorphin also has a role in cutaneous neurogenic inflammation and analgesia via endothelin-1 (ET-1) activation of keratinocytes leading to the release of β-endorphin.148 Similarly, CB seem to regulate the release of β-endorphin from keratinocytes thereby modulating nociception in the skin.149 These findings clearly indicate a crucial role for epidermally derived β-endorphin in cutaneous nociception. β-Endorphin may be also involved in the pathophysiology of acne150 and atopic dermatitis.151,152
β-Endorphin can be generated in human keratinocytes via activation of the μOR by ultraviolet radiation. In human melanocytes, β-endorphin has melanogenic, mitogenic, and dendritogenic effects in vitro, and expresses μOR.147 Accordingly, human hair follicle melanocytes also express β-endorphin and μOR, especially in glycoprotein-100-positive cells, suggesting a role of hair growth in an autocrine fashion. Thus, β-endorphin may be involved in pigmentation and hair growth. Recent findings indicate that β-endorphin also has a role in cutaneous neurogenic inflammation and analgesia via endothelin-1 (ET-1) activation of keratinocytes leading to the release of β-endorphin.148 Similarly, CB seem to regulate the release of β-endorphin from keratinocytes thereby modulating nociception in the skin.149 These findings clearly indicate a crucial role for epidermally derived β-endorphin in cutaneous nociception. β-Endorphin may be also involved in the pathophysiology of acne150 and atopic dermatitis.151,152
![]() Increased amounts of enkephalins were reported in lesional skin of psoriasis patients and were reduced in parallel with the clinical improvement induced by a topical vitamin D analog and a corticosteroid. Application of [met]-enkephalin induced a flare reaction in the skin that was by pretreatment with antihistamine, suggesting both histamine and histamine-independent involvement of enkephalins in neurogenic inflammation.153 [Met]-enkephalin induces infiltration of dermal lymphocytes, monocytes, and macrophages, and enkephalins protect against tissue damage caused by hypoxia, and inhibit differentiation and proliferation of keratinocytes.153,154 Thus, because enkephalins can modulate epidermal differentiation and inflammatory processes, these findings indicate that enkephalins may play a role in the pathogenesis of psoriasis.155
Increased amounts of enkephalins were reported in lesional skin of psoriasis patients and were reduced in parallel with the clinical improvement induced by a topical vitamin D analog and a corticosteroid. Application of [met]-enkephalin induced a flare reaction in the skin that was by pretreatment with antihistamine, suggesting both histamine and histamine-independent involvement of enkephalins in neurogenic inflammation.153 [Met]-enkephalin induces infiltration of dermal lymphocytes, monocytes, and macrophages, and enkephalins protect against tissue damage caused by hypoxia, and inhibit differentiation and proliferation of keratinocytes.153,154 Thus, because enkephalins can modulate epidermal differentiation and inflammatory processes, these findings indicate that enkephalins may play a role in the pathogenesis of psoriasis.155
![]() Dynorphin is a neurotransmitter as well as an immunomodulatory molecule. Immunohistochemical studies in animals revealed the presence of dynorphin A predominantly in sympathetic axons innervating the cutaneous venous bed, but also in sensory nerve fibers, Merkel cells, and immune cells within the inflamed tissue.156,157 The discovery of inhibited nociception during inflammation by endogenous opioids increased interest in this neuromodulatory peptide. Dynorphin may exert antipruritic effects via activation of κOR in the spinal cord.1 Finally, dynorphin modulates keratinocyte migration in wound healing.151
Dynorphin is a neurotransmitter as well as an immunomodulatory molecule. Immunohistochemical studies in animals revealed the presence of dynorphin A predominantly in sympathetic axons innervating the cutaneous venous bed, but also in sensory nerve fibers, Merkel cells, and immune cells within the inflamed tissue.156,157 The discovery of inhibited nociception during inflammation by endogenous opioids increased interest in this neuromodulatory peptide. Dynorphin may exert antipruritic effects via activation of κOR in the spinal cord.1 Finally, dynorphin modulates keratinocyte migration in wound healing.151
![]() Systemic158 as well as topical treatment159 with a μ-opioid receptor antagonist (systemically: naltrexone 50–100 mg; naltrexone cream 1%) has been shown to be effective for the therapy of atopic dermatitis and chronic eczema-associated pruritus.
Systemic158 as well as topical treatment159 with a μ-opioid receptor antagonist (systemically: naltrexone 50–100 mg; naltrexone cream 1%) has been shown to be effective for the therapy of atopic dermatitis and chronic eczema-associated pruritus.
![]() Melanocortins (MCs), adrenocorticotropin (ACTH), and melanocyte-stimulating hormones (α-, β-, γ-MSH) are derived from the precursor POMC after posttranslational processing by members of the prohormone convertase (PC) family.49 Initially, MCs were characterized as regulators of pigmentation and cortisol production but there is increasing evidence for a role of MSH in food intake, sexual behavior, exocrine gland function, and inflammation.49,160 MCs exert their effects by binding to melanocortin receptors (MC-R), which belong to the family of G-protein coupled receptors. The MC-R family includes five members (MC1-R to MC5-R), which display overlapping specificities and usually bind several MCs. Only MC2-R shows a strong selectivity for ACTH.161 MC-Rs, especially MC1-R, are more widely expressed than originally believed and occur not only on melanocytic and neuronal cells but also in various cells of the immune system, epithelial cells, endothelial cells, and fibroblasts49 (Table 102-1).
Melanocortins (MCs), adrenocorticotropin (ACTH), and melanocyte-stimulating hormones (α-, β-, γ-MSH) are derived from the precursor POMC after posttranslational processing by members of the prohormone convertase (PC) family.49 Initially, MCs were characterized as regulators of pigmentation and cortisol production but there is increasing evidence for a role of MSH in food intake, sexual behavior, exocrine gland function, and inflammation.49,160 MCs exert their effects by binding to melanocortin receptors (MC-R), which belong to the family of G-protein coupled receptors. The MC-R family includes five members (MC1-R to MC5-R), which display overlapping specificities and usually bind several MCs. Only MC2-R shows a strong selectivity for ACTH.161 MC-Rs, especially MC1-R, are more widely expressed than originally believed and occur not only on melanocytic and neuronal cells but also in various cells of the immune system, epithelial cells, endothelial cells, and fibroblasts49 (Table 102-1).
![]() The role of melanocortins in skin pigmentation is well established.162 Patients with POMC null mutations have recently been described to display the red hair and fair skin phenotype.163 Moreover, the inductive effects of UV light on melanocortins and MC1-R expression in epidermal cells indicates that tanning is apparently dependent on the activation of the MC system in the skin.164 α-MSH also stimulates follicular melanogenesis by preferentially increasing the synthesis of eumelanin over pheomelanin via the stimulation of tyrosinase activity.165 Furthermore, α-MSH reduces UVB-induced DNA damage not only through the induction of pigmentation but also by modulating the function of DNA repair molecules by inducing nuclear translocation of XPA, a critical factor controlling nucleotide excision repair signaling pathways.166 Recently a novel aspect involving the cytoprotective and antioxidative effect of α-MSH has been detected. Accordingly, α-MSH was found to prevent the UVB mediated suppression of Nrf transcription factors, which are crucially involved in the protection against oxidative stress.172 ACTH and α-MSH also may play a role in hair growth146 and the regulation of sebaceous gland function.167
The role of melanocortins in skin pigmentation is well established.162 Patients with POMC null mutations have recently been described to display the red hair and fair skin phenotype.163 Moreover, the inductive effects of UV light on melanocortins and MC1-R expression in epidermal cells indicates that tanning is apparently dependent on the activation of the MC system in the skin.164 α-MSH also stimulates follicular melanogenesis by preferentially increasing the synthesis of eumelanin over pheomelanin via the stimulation of tyrosinase activity.165 Furthermore, α-MSH reduces UVB-induced DNA damage not only through the induction of pigmentation but also by modulating the function of DNA repair molecules by inducing nuclear translocation of XPA, a critical factor controlling nucleotide excision repair signaling pathways.166 Recently a novel aspect involving the cytoprotective and antioxidative effect of α-MSH has been detected. Accordingly, α-MSH was found to prevent the UVB mediated suppression of Nrf transcription factors, which are crucially involved in the protection against oxidative stress.172 ACTH and α-MSH also may play a role in hair growth146 and the regulation of sebaceous gland function.167
![]() The best-investigated MSH in the skin is α-melanocyte-stimulating hormone (α-MSH). It is a tridecapeptide also derived from the POMC gene, but maturated by posttranslational processing. α-MSH has been demonstrated to be involved in pigmentation. In addition, it is a potent anti-inflammatory mediator when administered systemically or locally. This effect is mediated by receptor-mediated modulation of immune responses and by affecting skin cells such as fibroblasts. It regulates nuclear factor-κB (NF-κB) activation, the expression of adhesion molecules and chemokine receptors, and downregulates the production of proinflammatory cytokines and other molecules.168
The best-investigated MSH in the skin is α-melanocyte-stimulating hormone (α-MSH). It is a tridecapeptide also derived from the POMC gene, but maturated by posttranslational processing. α-MSH has been demonstrated to be involved in pigmentation. In addition, it is a potent anti-inflammatory mediator when administered systemically or locally. This effect is mediated by receptor-mediated modulation of immune responses and by affecting skin cells such as fibroblasts. It regulates nuclear factor-κB (NF-κB) activation, the expression of adhesion molecules and chemokine receptors, and downregulates the production of proinflammatory cytokines and other molecules.168
![]() Keratinocytes express MC-Rs and POMC and release α-MSH. α-MSH production by keratinocytes is increased by UV light and proinflammatory stimuli such as lipopolysaccharide (LPS) and IL-1. There is also evidence that the melanocortin system is involved in keratinocyte proliferation and differentiation. Since α-MSH has a variety of immunomodulatory and anti-inflammatory activities by downregulating the production of proinflammatory cytokines such as IL-1, IL-6, and TNF-α in several cell types and downregulating the expression of adhesion molecules on endothelial cells as well as inhibiting NF-kB activation. Very recent data indicate that the anti-inflammatory effects by H-bonds of α-MSH may be mediated via interaction of α-MSH with IL-1R1. Accordingly, an α-MSH related tripeptide, KdPT, was found to bind to IL-1R1 and one hydrophobic bond. α-MSH also induces IL-10 expression by human keratinocytes. These findings, together with the observation that systemic application of α-MSH blocks contact hypersensitivity (CHS) in mice and induces antigen-specific tolerance, indicate that α-MSH released in the skin after UV-irradiation may be one of the mediators responsible for UV-mediated immunosuppression.49,169 The role of α-MSH as mediator of inflammation is further strengthened by finding that α-MSH blocks cytokine and mediator release by mast cells and basophils.49 Moreover, α-MSH appears to be involved in the regulation of an immune response, since upon treatment with α-MSH, dendritic cells remain in an immature stage and induce regulatory T cells.170 There is recent evidence that α-MSH plays a crucial role in the development of cytotoxic CD8+ T cells and thereby contributes to major histocompatibility complex (MHC) class I restricted cytotoxicity.171
Keratinocytes express MC-Rs and POMC and release α-MSH. α-MSH production by keratinocytes is increased by UV light and proinflammatory stimuli such as lipopolysaccharide (LPS) and IL-1. There is also evidence that the melanocortin system is involved in keratinocyte proliferation and differentiation. Since α-MSH has a variety of immunomodulatory and anti-inflammatory activities by downregulating the production of proinflammatory cytokines such as IL-1, IL-6, and TNF-α in several cell types and downregulating the expression of adhesion molecules on endothelial cells as well as inhibiting NF-kB activation. Very recent data indicate that the anti-inflammatory effects by H-bonds of α-MSH may be mediated via interaction of α-MSH with IL-1R1. Accordingly, an α-MSH related tripeptide, KdPT, was found to bind to IL-1R1 and one hydrophobic bond. α-MSH also induces IL-10 expression by human keratinocytes. These findings, together with the observation that systemic application of α-MSH blocks contact hypersensitivity (CHS) in mice and induces antigen-specific tolerance, indicate that α-MSH released in the skin after UV-irradiation may be one of the mediators responsible for UV-mediated immunosuppression.49,169 The role of α-MSH as mediator of inflammation is further strengthened by finding that α-MSH blocks cytokine and mediator release by mast cells and basophils.49 Moreover, α-MSH appears to be involved in the regulation of an immune response, since upon treatment with α-MSH, dendritic cells remain in an immature stage and induce regulatory T cells.170 There is recent evidence that α-MSH plays a crucial role in the development of cytotoxic CD8+ T cells and thereby contributes to major histocompatibility complex (MHC) class I restricted cytotoxicity.171
![]() There is increasing evidence that α-MSH also modulates dermal fibroblast function since they have been shown to express MC1-R. Furthermore, a role of α-MSH as regulator of the extracellular matrix of the skin is supported by studies demonstrating an effect of α-MSH and collagen synthesis induced by TGF-β1, a key profibrotic cytokine implicated in the pathogenesis of fibrotic disorders including systemic sclerosis.172 The activity of α-MSH and its regulation of the collagen metabolism in conjunction with its anti-inflammatory activities could be of special value for the future therapy of fibrotic disorders.173
There is increasing evidence that α-MSH also modulates dermal fibroblast function since they have been shown to express MC1-R. Furthermore, a role of α-MSH as regulator of the extracellular matrix of the skin is supported by studies demonstrating an effect of α-MSH and collagen synthesis induced by TGF-β1, a key profibrotic cytokine implicated in the pathogenesis of fibrotic disorders including systemic sclerosis.172 The activity of α-MSH and its regulation of the collagen metabolism in conjunction with its anti-inflammatory activities could be of special value for the future therapy of fibrotic disorders.173
![]() Several lines of evidence indicate that melanocortins in the skin may function as part of the innate defense that ultimately protects the skin against environmental stress, infectious agents, and genotoxic stimuli. Accordingly, MCs induce melanogenesis to prevent the generation of skin cancer. α-MSH also was found to exhibit antimicrobial activity.174 Recently, it has been reported that α-MSH is able to protect melanocytes as well as keratinocytes from the most ubiquitous environmental stressor UV light via exerting an antiapoptotic activity.175 This may also explain the increased incidence of melanomas in patients with loss of function of MC1-R.49,176 Therefore, in the epidermis α-MSH may maintain the crucial pigment producing function of melanocytes and via reducing UVB-induced DNA damage, it may enhance genomic stability of both melanocytes and keratinocytes.
Several lines of evidence indicate that melanocortins in the skin may function as part of the innate defense that ultimately protects the skin against environmental stress, infectious agents, and genotoxic stimuli. Accordingly, MCs induce melanogenesis to prevent the generation of skin cancer. α-MSH also was found to exhibit antimicrobial activity.174 Recently, it has been reported that α-MSH is able to protect melanocytes as well as keratinocytes from the most ubiquitous environmental stressor UV light via exerting an antiapoptotic activity.175 This may also explain the increased incidence of melanomas in patients with loss of function of MC1-R.49,176 Therefore, in the epidermis α-MSH may maintain the crucial pigment producing function of melanocytes and via reducing UVB-induced DNA damage, it may enhance genomic stability of both melanocytes and keratinocytes.
![]() Anti-inflammatory effects of α-MSH have been confirmed in vivo using various animal models including irritant or allergic contact dermatitis, cutaneous vasculitis, asthma, inflammatory bowel disease, rheumatoid arthritis, and ocular and brain inflammation. Most of the anti-inflammatory activities of α-MSH can be attributed to its C-terminal tripeptide KPV, K(D)PT.177 Therapeutic potential has been also suggested in acne vulgaris178 or, for example, scleroderma.172
Anti-inflammatory effects of α-MSH have been confirmed in vivo using various animal models including irritant or allergic contact dermatitis, cutaneous vasculitis, asthma, inflammatory bowel disease, rheumatoid arthritis, and ocular and brain inflammation. Most of the anti-inflammatory activities of α-MSH can be attributed to its C-terminal tripeptide KPV, K(D)PT.177 Therapeutic potential has been also suggested in acne vulgaris178 or, for example, scleroderma.172