There are more than 2 dozen nerve entrapment syndromes in the body. Generally, these occur at sites of fibroosseous or fibromuscular tunnels. Any insult that leads to an increase in the size of the nerve or a decrease in the volume of the tunnel will cause compression. Resultant nerve ischemia sets off a cascade of events that lead to predictable clinical signs and symptoms. Here, we review the most common nerve entrapment syndromes and highlight their assessment and management. Specific clinical scenarios that require a high suspicion for nerve entrapment are highlighted.
Key points
- •
Nerve entrapment syndromes are a common cause of pain and disability.
- •
There are more than 2 dozen described nerve entrapment syndromes in the body.
- •
The mainstay of nonsurgical management includes activity modification, nerve gliding, and splinting.
- •
Surgical decompression involves releasing tight structures overlying the entrapped nerve.
Introduction
Nerve entrapment syndromes are common, and can lead to sensory disturbance, loss of motor function and pain. More than 2 dozen nerve entrapment syndromes have been described ( Table 1 ). Although they can occur acutely as a result of trauma with subsequent swelling, most compression neuropathies are chronic in nature. Chronic, focal compression of a nerve can occur as a result of ordinary, everyday activities, such as sleeping position or repetitive movements at work. Our understanding of these processes has evolved significantly over the past 40 years. Pain, although a common feature of nerve entrapment, has received less attention.
| Involved Nerve | Site of Entrapment | Signs and Symptoms | Surgical Decompression |
|---|---|---|---|
| Upper extremity | |||
| Median nerve | Carpal tunnel | Pain and paraesthesias in the radial 3 and one-half digits (sparing of PCM) and night-time wakening APB weakness , muscle atrophy | Release of transverse carpal ligament |
| Median nerve | Forearm | Volar forearm pain exacerbated by pronation and reproduced with supination and pressure over leading edge of pronator Paresthesias and sensory loss in median nerve distribution including PCM Weakness of muscles supplied by AIN ( FPL, FDP [1, 2], PQ ) | Release of lacertus fibrosis, deep head of pronator, flexor digitorum superficialis arch with or without ligament of Struthers with or without supracondylar process |
| Ulnar nerve | Cubital tunnel | Pain and paraesthesias in ring and small fingers Aching medial elbow into forearm Clumsiness and weak grip strength Atrophy first dorsal interossei, abductor digiti minimi | Release of cubital tunnel, heads of FCU, with or without arcade of Struthers with or without anconeus with or without transposition |
| Ulnar nerve | Guyon’s canal | Pain and paraesthesias in ring and small fingers Clumsiness, clawing of ring and small fingers Atrophy first dorsal interossei, abductor digiti minimi | Release of antebrachial fascia, Guyon’s canal, leading edge of hypothenar muscles |
| Radial nerve | Spiral groove | Radial nerve palsy ( weakness/loss of wrist/elbow and finger/thumb extension ), numbness dorsoradial aspect of hand | Neurolysis of radial nerve as travels around humerus in spiral groove |
| Radial nerve | Radial tunnel | Palsy of muscles supplied by posterior interosseous nerve (finger, thumb extensors) Arm fatigue and pain dorsal forearm , worsens with elbow extension and forearm rotation | Release of ECRB, Arcade of Frohse, with or without radial recurrent vessels (leash of Henry) |
| Superficial sensory branch of the radial nerve | Forearm: between brachioradialis, ECRL | Paraesthesias dorsoradial aspect of the hand , exquisite pain with ulnar flexion of the wrist or activities such as gripping and pinching | Release (with or without resection) of brachioradialis tendon, neurolysis of superficial sensory branch of the radial nerve |
| Axillary nerve | Quadrangular space | Pain posterior shoulder , weakness shoulder abduction , numbness lateral arm , point tenderness over quadrangular space | Release of lateral head of triceps tendon with or without teres minor fascia, neurolysis of axillary nerve |
| Suprascapular nerve | Suprascapular notch | Deep, diffuse pain posterior and lateral shoulder that may refer down the arm, to the neck, or to upper anterior chest wall Muscle weakness and atrophy of supraspinatus and infraspinatus (external rotation) | Release of transverse scapular ligament, which forms the roof of the suprascapular notch |
| Brachial plexus | Thoracic outlet | Proximal pain (shoulder/scapula) with distal numbness with or without paresthesias (usually C8/T1 distribution) Symptoms brought on with activity/overhead movement | Anterior and middle scalenectomy with or without rib resection |
| Lateral antebrachial cutaneous nerve | Arm: biceps tendon | Pain, paresthesias lateral forearm | Neurolysis of the lateral antebrachial cutaneous nerve |
| Lower extremity | |||
| Femoral nerve | Iliacus fascia, inguinal ligament | Weakness/atrophy hip flexion, knee extension , paraesthesias anteromedial thigh, medial leg, gait disturbance | Neurolysis femoral nerve, with or without (partial) release of inguinal ligament |
| Lateral cutaneous nerve of the thigh | Inguinal ligament (meralgia paresthetica) | Pain, paresthesias anterolateral thigh , exacerbated by standing, walking; relief with nerve block | Neurolysis of lateral femoral cutaneous nerve along its course |
| Obturator nerve | Medial thigh | Pain and paresthesias medial thigh, weakness of thigh adduction, internal rotation , gait disturbance; relief with nerve block | Release of adductor brevis fascia |
| Common peroneal nerve | Fibular neck | Pain and paraesthesias in lateral leg and foot , weakness dorsiflexion, foot drop | Release of 3 intermuscular septal planes of the lateral and anterior compartments |
| Superficial peroneal nerve | Distal third of leg | Pain and paraesthesias in lateral leg and foot | Release of deep fascia overlying superficial peroneal nerve |
| Deep peroneal nerve | Dorsum of foot | Weakness, pain, and paresthesias of the foot and ankle, specifically, in the first web space | Release of extensor retinaculum over anterior ankle, with or without release of extensor hallucis brevis |
| Posterior tibial nerve | Soleus arch | Calf pain, exacerbated by exercise | Release of tendinous leading edge of soleus |
| Posterior tibial nerve | Tarsal tunnel | Pain and paresthesias over medial ankle and heel , sole of the foot , and toes ; and weakness and atrophy of toe flexion muscles | Release of the flexor retinaculum, as well as the superficial and deep fascia of the abductor muscle belly |
| Saphenous nerve | Adductor canal Gonyalgia paresthetica, minor causalgia | Deep aching pain medial thigh and knee, paresthesias medial leg and foot ; relief with nerve block | Release of adductor canal |
| Plantar nerves | Morton’s neuroma: between metatarsal heads | Pain in the webspace with walking ; pain shoots into toes and is relieved at rest and with elevation; lateral web spaces more commonly involved | Release of deep transverse metatarsal ligament and overlying fascia |
| Head and neck | |||
| Supraorbital and supratrochlear nerves | Supraorbital/frontal | Chronic headaches/migraines in frontal region ; relief with injection of botulinum toxin or nerve block | Release of supraorbital notch/foramen, excision corrugator muscle with or without excision of supraorbital/trochlear vasculature |
| Zygomaticotemporal and auriculotemporal nerve | Temporal | Chronic headaches/migraines in temporal region ; relief with injection of botulinum toxin or nerve block | Release deep fascial bands with or without release of temporalis muscle with or without ligation superficial temporal artery |
| Greater, lesser and third occipital nerves | Occipital | Chronic headaches/migraines in occipital region ; relief with injection of botulinum toxin or nerve block | Release of semispinalis capitis muscle, with or without trapezial tunnel plus sternocleidomastoid fascia/muscle |
| Sphenopalatine ganglion | Nasal | Chronic headaches/migraines in frontal region; relief with injection of botulinum toxin or nerve block | – |
Here, we discuss the pathophysiology of nerve entrapment and its relationship to pain. We review the most common upper and lower extremity nerve entrapment syndromes and highlight their assessment and management. We also discuss several clinical scenarios where nerve entrapment is underdiagnosed. With a strong foundation and understanding of the biology of compression neuropathy, indications for surgical decompression will continue to expand to provide relief for patients suffering from pain.
Microanatomy
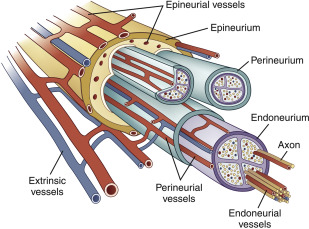
Peripheral nerves are composed of motor sensory, and autonomic fibers. Type A fibers are large myelinated nerve fibers and include afferent and efferent motor and sensory fibers. They exhibit the highest conduction velocity. Type B fibers are smaller myelinated fibers, and are made up of preganglionic autonomic nerve fibers. Type C fibers are the thinnest and unmyelinated, and include visceral and somatic pain fibers, as well as postganglionic autonomic nerve fibers. The fibers (known as axons), are grouped into fascicles, which are bundled with blood vessels and connective tissue to form the nerve ( Fig. 1 ). There are 3 layers of connective tissue matrix. The innermost layer, the endoneurium, surrounds axons and Schwann cells and is resistant to injury by stretch. The middle layer, the perineurium, surrounds fascicles and maintains the physiologic milieu and the blood–nerve barrier. The external layer, the epineurium, provides structural support and protects against compression ; it is most abundant where a nerve crosses a joint. The blood supply is 2-fold: intrinsic vessels run within the endoneurial space, and anastomose with the extrinsic plexus of the epineurial space.
Pathophysiology of nerve entrapment
Nerve entrapment occurs at sites of fibroosseous or fibromuscular tunnels. Owing to the limited volume within these tunnels, any insult that leads to an increase in the size of the nerve or a decrease in the volume of the tunnel will cause compression of the nerve and affect the intrinsic and extrinsic blood flow. Pressure increases of as little as 20 mm Hg can lead to venous stasis and subsequent extraneural edema; at 80 mm Hg, all intraneural blood flow ceases. Stretching of nerves can also lead to venous stasis. When a nerve is stretched by 8% of its resting length, venous outflow is blocked. Over time, venous outflow obstruction leads to fibrosis and scarring, which further worsens endoneurial edema. , The intact perineurium prevent this increased pressure from being relieved, causing a miniature compartment syndrome within the nerve. The resultant hypoxia leads to further inflammation, fibrosis, demyelination, and eventually, axonal degeneration. ,
These intraneural physiologic changes are accompanied by predictable clinical symptoms. Patients with mild or intermittent nerve entrapment experience intermittent paraesthesias, aching and weakness in the distribution of the affected nerve. These symptoms are usually relieved by a change in position or the shaking out one’s hand or arm. However, as neural hypoxia becomes severe or chronic in nature, symptoms progress to constant paraesthesias, atrophy, and pain.
Pain in nerve entrapment
There are 4 proposed mechanisms for neuropathic pain, which may explain the pain phenomena of nerve entrapment: denervation, ectopic activity, peripheral sensitization, and central sensitization. With partial nerve damage, pain can occur as a result of injured and uninjured afferents supplying the affected region. Damaged afferent pathways are responsible for sensory loss owing to denervation, but can also contribute to ongoing pain from ectopic activity in the dorsal root ganglia that results from the loss of trophic support. Undamaged afferent nociceptors, in contrast, can be peripherally sensitized by the inflammatory process (macrophage infiltration, T-cell activation, increased expression of proinflammatory cytokines) that ensues after nerve damage, and cause hyperalgesia from enhanced trophic support in the dorsal root ganglia. , Both input from damaged and undamaged nociceptors can produce central sensitization, which can also cause hyperalgesia. Ongoing pain may be the only manifestation of nerve entrapment (owing to ectopic activity), but in many cases altered perception to evoked pain (hyperalgesia owing to denervation, peripheral sensitization, and central sensitization) may be present.
Nerve entrapment causes a disturbance of fast axonal transport along the nerve in the area of compression, and leads to ectopic expression of the transduced proteins, which is thought to result in the Tinel sign, where patients experience paraesthesias or dysesthesias when the area over the irritated nerve is tapped. An increase in substance P immunoreactivity has been noted in the dorsal root ganglia after nerve injury, as well as at sites of nerve entrapment, and has been proposed as the cause of the reflexive collapse response noted in the scratch collapse test.
Acute versus chronic nerve entrapment
There are 2 key differences in acute traumatic compression neuropathy and chronic compression neuropathy. First, axonal degeneration is noted early in acute nerve entrapment, although it is a late finding in chronic nerve entrapment. Histologically, axonal degeneration in chronic nerve entrapment is not visualized until muscle atrophy is clinically evident. Instead, chronic nerve entrapment first results in demyelination and slowing of nerve conduction, followed by subsequent thinner remyelination. Second, Schwann cell proliferation in chronic compression is independent of macrophages and instead is induced by mechanical forces. In contrast, acute entrapment shows greater infiltration of macrophages into the nerve.
The double crush
Upton and McComas first introduced the theory of the double crush phenomenon in 1973. They proposed that compression of a nerve at a proximal point will compromise axoplasmic flow, thus making the same nerve increasingly susceptible to injury at another, more distal point. For example, a patient with cervical radiculopathy or thoracic outlet syndrome may develop carpal tunnel syndrome (CTS) more easily. In 2004, Lundborg described the reverse double crush syndrome, in which a site of distal nerve entrapment predisposes the nerve to more proximal compression injury. He hypothesized that compression of a nerve at a distal site would decrease flow of neurotrophic substances back to the neuron body, with subsequent alteration of axoplasmic flow. Both theories suggest that entrapment of a nerve can affect axoplasmic transport and render other areas of the nerve more susceptible to entrapment.
Median nerve entrapment
Carpal Tunnel Syndrome
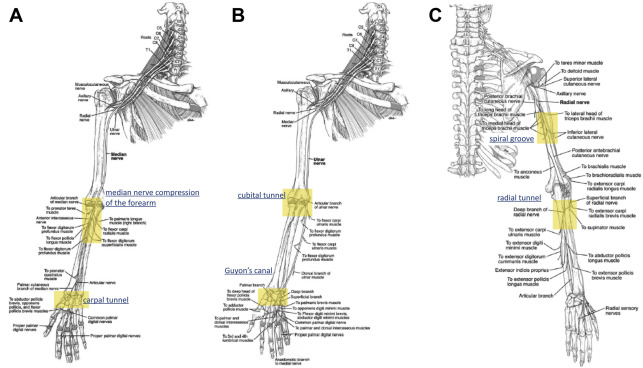
The median nerve (C6–T1) can become compressed at multiple points along its course from the distal humerus to the wrist ( Fig. 2 ). The most common site of entrapment is at the carpal tunnel, where the nerve travels under the transverse carpal ligament. With a prevalence ranging from 3.72% to 6.80% in the United States, CTS is the most common nerve entrapment syndrome in the body. Classic symptoms of CTS include intermittent pain and parenthesis in the radial three and a half digits and night-time wakening. Symptoms are often exacerbated by gripping and certain hand positions; symptoms are relieved by shaking out the hand. On examination, patients exhibit decreased sensation in the median nerve distribution with sparing of the palmar cutaneous nerve distribution over the thenar eminence. Provocative tests include the Tinel test, the Phalen test, the median nerve compression test, and recently the scratch collapse test. Over time, paraesthesias progress to become constant. In late CTS, weakness and thenar muscle atrophy are noted. Diagnosis can usually be made by history and physical examination alone. If examination is equivocal, electrodiagnostic studies (EDX) can be helpful in establishing the diagnosis. A negative EDX, however, does not preclude the diagnosis of CTS. EDX only evaluates large myelinated fibers, and will thus be normal in mild or early CTS, when only small or unmyelinated nerve fibers are affected.