(1)
Department of Otolaryngology, Cleveland Clinic, Head and Neck Institute, Cleveland, OH, USA
Abstract
Either iatrogenic or traumatic peripheral nerve injuries, which result in significant morbidity for patients, often require interposition nerve autografts. However, nerve autografts do have acknowledged limitation including donor availability, potential donor site morbidity including scarring, sensory loss, and painful neuroma formation. Furthermore, there can be inconsistent and suboptimal outcomes for patients with autograft repairs. This led to the development of nerve allograft transplantation models under the guidance of Dr. Maria Siemionow at the Cleveland Clinic Foundation. It was hypothesized that specific targeting of T cells with anti-αβ TCR mAbs in combination with CsA, which curbs nonspecific cell-mediated rejection via inhibition of the IL-2 receptor, would modulate the nerve allograft response to prevent rejection and afford successful nerve regeneration. A rat animal model with varying immunocompatibility barriers was proposes.
We were able to demonstrate improved functional, electrophysiologic, and histomorphometric outcomes in animals treated with anti-αβ TCR mAbs combined with CsA after nerve allograft transplantation when compared to animals receiving no treatment and CsA alone.
Nerve allograft transplantation modeling offers great promise to 1 day realize the goal of personalized medical care for those afflicted with peripheral nerve injuries. Models to improve immunodulating regimens, provide new allograft constructs, and new methods of monitoring the rejection process will allow for improved care of patients as these studies are translated into clinical care.
Keywords
NerveTransplantationAllograftImmunomodulationNerve injuryAlthough clinical advances in reconstruction strategies have continued to evolve over the years, there continues to be a limitation in functional reconstitution for patients afflicted with traumatic injuries. Both iatrogenic or traumatic peripheral nerve injuries, which result in significant morbidity for patients, often require interposition nerve autografts [1]. However, nerve autografts do have acknowledged limitation including donor availability, potential donor site morbidity including scarring, sensory loss, and painful neuroma formation [2]. Furthermore, there can be inconsistent and suboptimal outcomes for patients with autograft repairs. This led to the development of nerve allograft transplantation models under the guidance of Dr. Maria Siemionow at the Cleveland Clinic Foundation. This alternative model approach using nerve allografts does have potential drawbacks including viral transmission and the need for transient host immunosuppressive therapy. Allografts do offer the possibility to serve as a scaffold across which recipient native nerve may regenerate [3]. The development of an effective immunomodulating regimen was central to our strategy in allograft transplantation modeling. It was hoped that the immunomodulating regimen would be required only until the regeneration was complete to prevent the sequelae of immunosuppression including late opportunistic infections and malignancy. Therefore, we pursued a model that will be described within this chapter including experimental results and a current review of select allograft models that have been pursued at other institutions.
We investigated a short term combined protocol of anti-αβ T-cell receptor monoclonal antibodies (anti-αβ TCR mAbs) and cyclosporine (CsA) to induce tolerance and allow for regeneration. The rationale for this protocol was based on prior work in the laboratory demonstrating that a 5 week protocol of anti-αβ TCR mAbs in combination with CsA could be successfully used to induce tolerance in rat hind limb allograft transplantation across major histocompatibility barriers [4]. It was hypothesized that specific targeting of T cells with anti-αβ TCR mAbs in combination with CsA, which curbs nonspecific cell-mediated rejection via inhibition of the IL-2 receptor, would modulate the nerve allograft response to prevent rejection and afford successful nerve regeneration [5].
Allotransplant Model
The experimental animal model is outlined in Table 67.1 and essentially consisted of Lewis, Lewis-Brown Norway, and Brown – Norway rats to create a model with animals displaying a continuum of major histocompatibility mismatches. This afforded an interesting semiallogeneic nerve transplant model. A rat sciatic nerve transplantation model was employed using 1.5 cm sciatic nerve deficits that were created and repaired with microsurgical technique with ten interrupted nylon epineural sutures (Fig. 67.1). The animals were assessed at 6 and 12 weeks to evaluate their functional recovery, both clinical and electrophysiologic. Further histologic and morphometric analysis was performed at the time of euthanasia (Table 67.1).
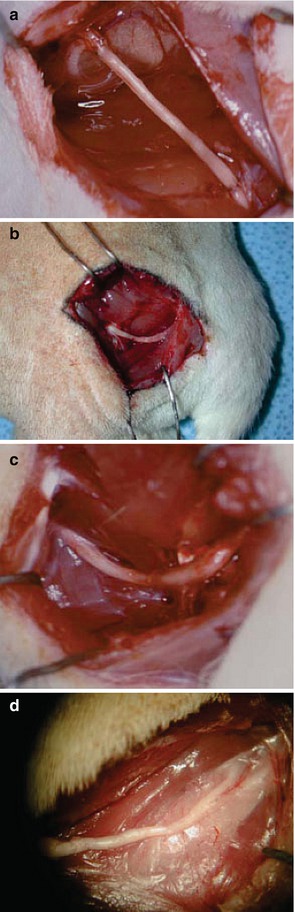
Table 67.1
Experimental design
Group | N | Recipient | Donor | Treatment |
|---|---|---|---|---|
1 | 6 | Lewis | Lewis | No treatment |
2 | 5 | Lewis | LBN | No treatment |
3 | 5 | Lewis | BN | No treatment |
4 | 7 | Lewis | LBN | CsA 5 weeks |
5 | 5 | Lewis | LBN | CsA/anti αß TCR mAbs, 5 weeks |
6 | 5 | Lewis | BN | CsA/anti αß TCR mAbs, 5 weeks |
7 | 5 | Lewis | LBN | CsA/anti αß TCR mAbs, 1 week |

Fig. 67.1
Operative pictures of allografts (a) LBN allograft initially placed in Lewis rat, (b) LBN-LEW transplant treated with 5 weeks of combined immunomodulation protocol at 12 weeks, (c) LEW-LEW isograft at 12 weeks, and (d) BN-LEW allograft with no treatment at 12 weeks









