Needle Localization Biopsy of Nonpalpable Breast Lesions
Virginia M. Herrmann
The increasing use of screening, and in particular digital mammography, ultrasound, and breast magnetic resonance imaging (MRI), has resulted in earlier detection of breast cancer (1,2). Screening mammography has improved significantly in the last two decades. This, coupled with emphasis on breast screening and guidelines recommending annual mammography for women 40 years of age and older, has contributed to the increased detection of small, nonpalpable lesions. The early detection of nonpalpable lesions led to the development of breast needle localization (BNL) to obtain a tissue diagnosis. Mammographic detection of lesions requiring BNL include a nonpalpable mass or density within, but distinct from normal breast parenchyma, architectural distortion, and clustered calcifications. These lesions are frequently discovered during screening mammography, and in most screening programs, approximately 15% of all lesions detected are malignant (3). The diagnosis of breast cancer at a very early stage has resulted in improved overall and disease-free survival for patients.
History and Rationale
The technique of BNL for nonpalpable lesions was first described by Dodd in 1966 (4,5). Nonpalpable lesions were localized by using a stiff needle placed near the lesion. This method was associated with several problems, including movement or dislodgement of the stiff needle during the period between its placement in radiology and transporting the patient to the operating room, movement of the needle during compression mammography, and movement or dislodgment of the needle during surgery. Inadequate tissue sampling was a frequent result.
Subsequent reports described the use of a combination of a needle and hooked wire with excellent accuracy and low biopsy failure rates. The wire was recommended to be placed within 1 mm of the lesion and secured within the breast tissue (6,7). In this method, the needle is removed, leaving the wire in place, with the wire hook placed within or immediately adjacent to the lesion. The hooked wire makes removal or dislodgment of the wire less likely than with a rigid needle.
BNL of suspicious nonpalpable lesions has been shown to be a reliable method for obtaining a diagnosis and has been the standard of care for the past several decades (8). The procedure can be performed by using local anesthesia and has been associated with a less than 2% chance of a missed lesion and a relatively low complication rate, similar to excisional biopsy of palpable lesions (9).
And while BNL of nonpalpable lesions is highly accurate, it can still be associated with some failures in obtaining the lesion seen on imaging. Whether the procedure was performed for diagnostic or therapeutic purposes, a review indicates the overall miss rate of this procedure is between 0% and 18% (mean 2.6%) and a cancer miss rate or false-negative BNL is 0% and 8% (mean 2.0%) (9,10,11).
BNL generally requires the patient undergo a separate procedure (wire or needle localization) in a separate department (radiology) from the operating room, where the lesion is removed. This technique is also associated with some morbidity and complications, to include pain and anxiety during needle placement, dislodgment of the needle or wire prior to operative removal, displacement of the wire when the breast is taken out of compression, and transecting the wire during surgery. The procedure requires significant anesthesia (e.g., monitored anesthesia care with intravenous sedation and local anesthetic, or general anesthetic) and has the potential for causing scar or distortion on subsequent mammography. Additional problems include excision of excess amount of tissue if the needle localization is not ideally centered in the lesion, resulting in possible cosmetic defect.
Historically, BNL performed as the primary procedure has been associated with the frequent need for a second operation if malignancy is found. When BNL was performed as the initial procedure and malignancy was found, the incidence of positive margins ranged from 55% to 83% (12,13).
With the development of stereotactic and MRI-guided biopsy techniques, the need for BNL of nonpalpable lesions has decreased. Stereotactic, ultrasound-guided, and MRI-guided biopsy have allowed the pathologic confirmation of benign lesions, obviating the need for surgery in many patients. Open excisional biopsy of many or most benign lesions is probably not necessary.
Despite the advances in image-guided, minimally invasive biopsy for breast lesions, BNL continues to have an important role in the management of patients with nonpalpable breast lesions. Indications for this procedure include the following:
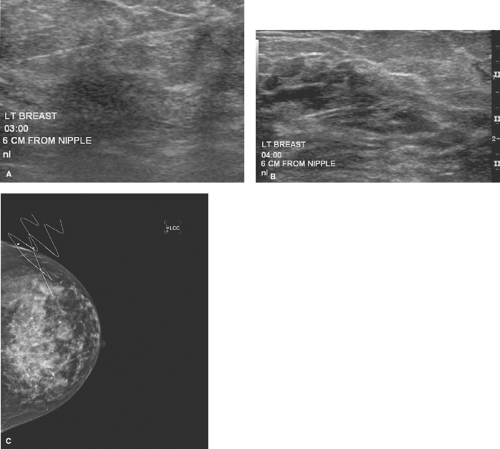
Suspicious microcalcifications or solid lesions located too close to the chest wall to allow safe stereotactic core biopsy are generally considered for BNL. Ultrasound localization of lesions close to the chest wall is often a preferred and safer method of BNL (Fig. 7.1).
Calcifications or papillary lesions in the immediate retroareolar area are often difficult to biopsy with image-guided technique and may be more amenable to BNL (Fig. 7.2).
Atypical hyperplasia (ductal or lobular) found on core biopsy, and not associated with a palpable lesion, generally requires BNL because of the high incidence of ductal carcinoma in the associated tissue.
Nonpalpable papillary lesions found on core biopsy should be excised with BNL to exclude papillary carcinoma.
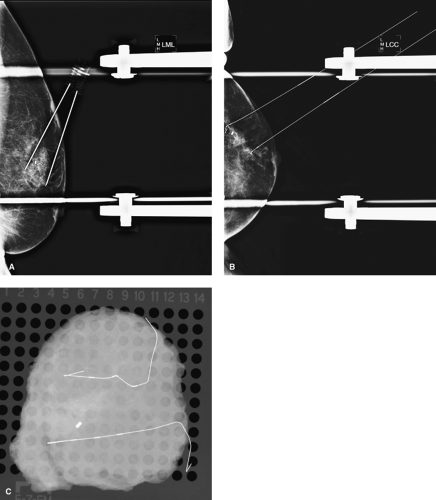
Radial scar, diagnosed either by mammogram or by core biopsy, should be totally excised by using BNL. These lesions, even if benign on core biopsy, are associated with a significant incidence of associated atypia or ductal carcinoma in situ (DCIS), necessitating complete excision (Fig. 7.3).
BNL is the procedure of choice if breast conservation therapy is anticipated for patients with nonpalpable cancers and a prior core biopsy confirming malignancy (Figs. 7.4 and 7.5).
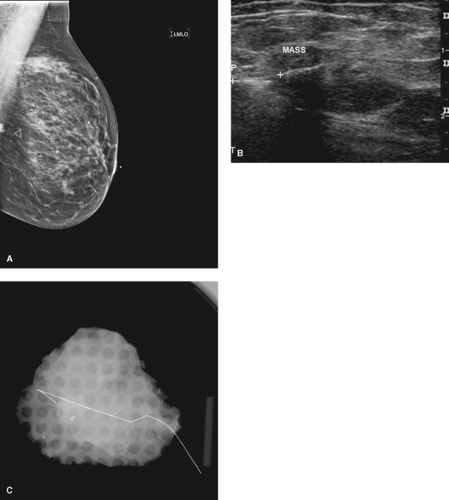
Figure 7.1 A. Lesion seen posteriorly near chest wall on mammogram. B. Lesion seen on ultrasound. C. Lesion successfully removed by using ultrasound localization.
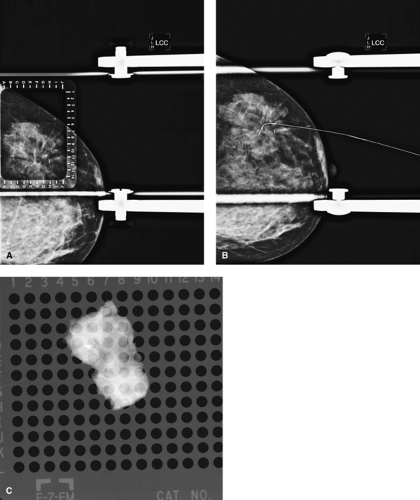
Figure 7.3 A. Radial scar on mammogram with clip from previous core biopsy. B. Mammographic breast needle localization of radial scar and clip. C. Specimen radiograph with clip and radial scar.
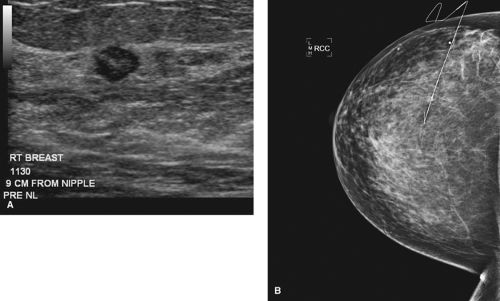
Figure 7.4 A. Pre–breast needle localization (BNL) of cancer seen on ultrasound. B. Mammogram of BNL showing small, nonpalpable cancer.
BNL is used to localize clip placement, or the “epicenter” of the tumor or tumor bed in patients treated with neoadjuvant chemotherapy. These patients ideally have had a clip or marker placed before commencing neoadjuvant chemotherapy, which localizes the area of cancer. Following treatment, the clip may be the only sign of the previously noted malignancy (Fig. 7.6).
Technical Considerations
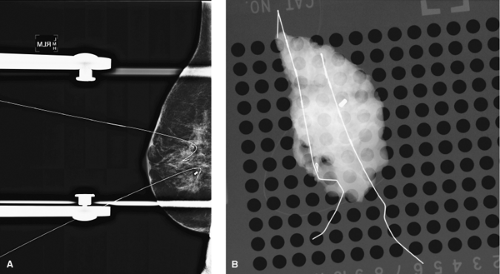
Radiologists and surgeons have refined the technique of BNL to allow more accurate localization of breast lesions. The use of various needle or wire lengths allows access
to lesions that may be deep in the breast or access to lesions in patients with large breast volume (Fig. 7.7). There are several needles used to localize lesions, most commonly, the Kopan needle, the Homer needle (J-wire), and the Hawkins retractable wire.
to lesions that may be deep in the breast or access to lesions in patients with large breast volume (Fig. 7.7). There are several needles used to localize lesions, most commonly, the Kopan needle, the Homer needle (J-wire), and the Hawkins retractable wire.
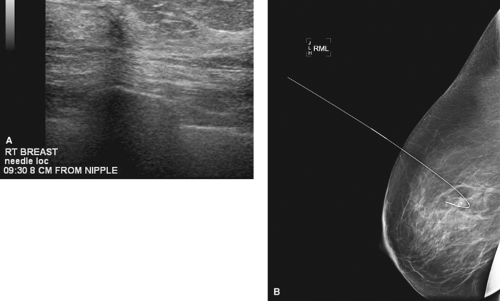 Figure 7.5 A. Small invasive tubular carcinoma on ultrasound. B. Mammographic breast needle localization of tubular invasive carcinoma. |
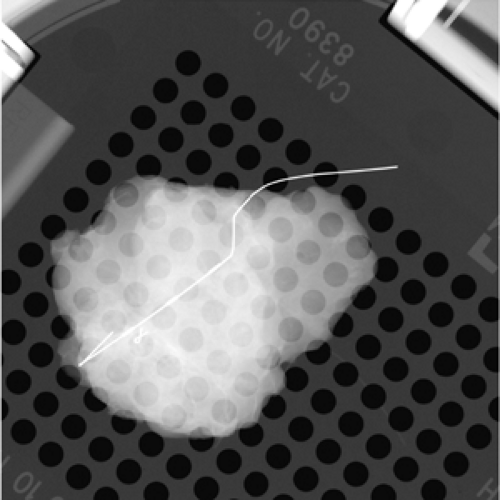 Figure 7.6 Specimen radiograph confirming clip in small tumor bed. Patient treated with neoadjuvant chemotherapy. |
Image-guided BNL can be performed by using mammography, ultrasound, or MRI. The decision of which imaging modality to use for BNL is largely determined by the following:
the type of lesion,
how the lesion is best visualized, and
available equipment and expertise in any given institution.
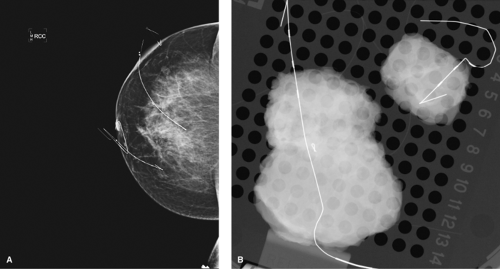 Figure 7.7 A. Two lesions localized with different lengths of wire. B. Specimen radiograph of each lesion with wire. |
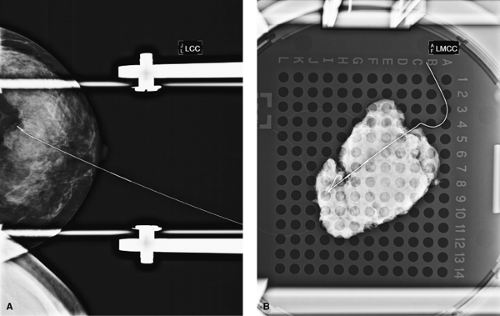 Figure 7.8 A. Breast needle localization of calcifications. B. Specimen radiograph confirming calcifications in specimen. |
The decision of which modality to use is generally suggested by the radiologist, often with input from the surgeon.
Mammography
Breast microcalcifications are often best biopsied by using mammographic BNL (Fig. 7.8). A needle containing a wire is inserted into the breast. The needle is removed, and the wire hook “anchors” the wire in the breast lesion. A variable length of wire will extend beyond the skin. The wire tip should ideally be placed within the lesion or within 1 mm of the lesion and be well anchored in the breast tissue. Studies have indicated a failure rate of 2% to 4% if the wire is placed within 1 to 3 cm of the lesion (7). Generally, radiologists prefer to enter the skin parallel to the chest wall and approach the lesion from above (Fig. 7.9). A postlocalization mammogram is performed and should be available to the surgeon in the operating room as a visual guide to assess the depth of the lesion and
needle, the proximity of the needle tip to the lesion, and the extent of the lesion. The increasing availability and use of digital mammography has expedited BNL. Recent data have demonstrated that full-field digital mammography is superior to screen–film mammography in the time needed for BNL (14). The time needed to position the patient and place the needle was shorter with digital mammography than screen–film mammography. In addition, digital mammography may provide superior image quality, reduced radiation exposure, and fewer delays as film processing is unnecessary.
needle, the proximity of the needle tip to the lesion, and the extent of the lesion. The increasing availability and use of digital mammography has expedited BNL. Recent data have demonstrated that full-field digital mammography is superior to screen–film mammography in the time needed for BNL (14). The time needed to position the patient and place the needle was shorter with digital mammography than screen–film mammography. In addition, digital mammography may provide superior image quality, reduced radiation exposure, and fewer delays as film processing is unnecessary.
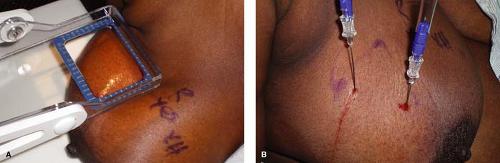 Figure 7.9 A. Lesion approached from above for breast needle localization (BNL). B. BNL completed with lesion approached from above. |
Prior to BNL, the surgeon should review the radiographs with the radiologist to provide a clear understanding of the lesion(s) to be localized and the imaging method to be used for wire placement (e.g., ultrasound-, mammogram-, or MRI-guided biopsy).
Bracketed needle localization is particularly helpful in certain circumstances. Patients with extensive microcalcifications may not be ideal candidates for stereotactic core biopsy. More commonly, however, bracketed needle localization is used to identify the extent of calcifications in a patient with core biopsy-proven DCIS (Fig. 7.10). The
extent of calcifications may underestimate the extent of DCIS; therefore, every attempt should be made to remove all of the suspicious calcifications with adequate margins. In bracketed needle localization, a wire is placed generally at the anterior and the posterior extent of calcifications. Two or more wires may be used to “bracket” the area of concern. Bracketed needle localization is also helpful to identify two or more lesions in the same quadrant (i.e., multifocal) lesions (Fig. 7.11). Two or more lesions can be removed through one incision. If the lesions are biopsy-proven or suspected to be malignant, both lesions can be removed along with the tissue between the two lesions and an adequate margin (Fig. 7.12).
extent of calcifications may underestimate the extent of DCIS; therefore, every attempt should be made to remove all of the suspicious calcifications with adequate margins. In bracketed needle localization, a wire is placed generally at the anterior and the posterior extent of calcifications. Two or more wires may be used to “bracket” the area of concern. Bracketed needle localization is also helpful to identify two or more lesions in the same quadrant (i.e., multifocal) lesions (Fig. 7.11). Two or more lesions can be removed through one incision. If the lesions are biopsy-proven or suspected to be malignant, both lesions can be removed along with the tissue between the two lesions and an adequate margin (Fig. 7.12).
Specimen radiography is strongly recommended following needle localization of suspicious breast lesions. The incidence of false-positive specimen radiographs may be as high as 7.8% and the false-negative rate as high as 55% (15). Hasselgren et al. (15




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree