Fig. 48.1
Myxoid melanoma. Melanocytes are embedded in a myxoid stroma, composed of basophilic, acidic mucin, that may be either focal or diffuse
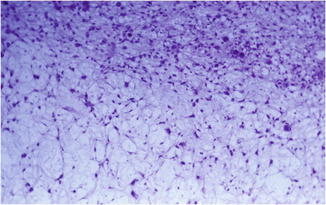
Fig. 48.2
Myxoid melanoma. Spindle, round, stellate-shaped, and atypical melanocytes embedded in a myxoid stroma
Distinction of primary from secondary metastatic MMM is based on the demonstration in the primary lesions of an atypical intraepidermal melanocytic proliferation (Fig. 48.3). Myxoid metastasis of melanoma is usually associated with a primary neoplasm that does not manifest a myxoid morphology.
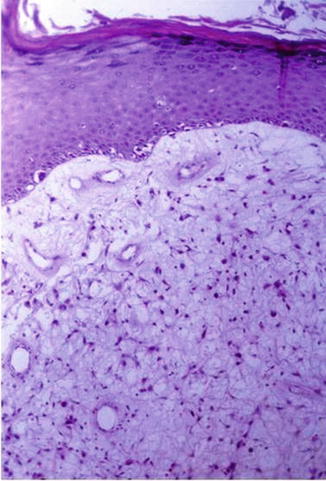

Fig. 48.3
The pattern of intraepidermal melanocyte involvement can be subtle, with only focal irregular proliferation in the lower layer overlying a myxoid proliferation
Immunohistochemistry analysis of the tumor shows uniform staining of S-100, while staining with HMB 45 or melan-A can be negative. Colloidal iron or Alcian blue at low pH detects mucin in the stroma.
Differential Diagnosis
Histologically, MMM should be differentiated from other epithelial mucin-containing tumors such as metastatic adenocarcinomas, and malignant sweat duct tumors, and from (mesenchymal) mucin-containing soft tissue tumors such as low-grade fibromyxoid sarcoma, low-grade myofibroblastic sarcoma, myxoid dermatofibrosarcoma protuberans, myxoid liposarcoma, extraskeletal myxoid chondrosarcoma, metastatic chordoma, myxoid rhabdomyosarcoma, myxoid synovial sarcoma, myxoid follicular dendritic cell sarcoma, nerve sheath myxoma, neurothekeoma, and malignant melanotic schwannoma. The malignant neoplasms of the sweat gland and duct manifest keratin positivity in most cases, as do metastatic carcinomas such as those of the breast and lung. Furthermore, these tumors usually express epithelial mucin and not mucin of mesenchymal origin. Low-grade fibromyxoid sarcoma and low-grade myofibroblastic sarcoma are generally S-100 negative. Myxoid dermatofibrosarcoma protuberans is CD34 negative, unlike its conventional counterpart, but can be distinguish by the lack of S-100 protein expression. Moreover, it stains positively for SMA. Liposarcoma rarely presents as a primary skin neoplasm and usually has plexiform, delicate “chicken-wire” vasculature that is lacking in MMM. Moreover, liposarcomas manifest weak cytoplasmic membrane rim staining with S-100 protein quite unlike that seen in a melanocytic neoplasm. Extraskeletal myxoid chondrosarcoma is a malignant mesenchymal neoplasm of uncertain differentiation (despite its name, there is no evidence of cartilaginous differentiation). It has a multinodular architecture defined by fibrous septa that divide the tumor into hypocellular lobules with myxoid matrix. It is weakly positive for S-100 in only a minority of cases, and it is characterized by NR4A3 (9q22) gene rearrangement. In chordoma, a malignant tumor showing notochordal differentiation, the cells are separated into lobules by fibrous septa and manifest spongelike or bubbly cytoplasmic vacuolation (physaliphorous cells). The expression of S-100 protein and cytokeratins enables distinction from MMM. Rhabdomyosarcoma characteristically is desmin positive and S-100 negative, unlikely melanoma. Synovial sarcoma rarely presents as a primary skin neoplasm. Around half of such tumors express S-100 protein, whereas most display epithelial differentiation (epithelial membrane antigen and/or cytokeratins expression), and most have the translocation t(X;18)(p11;q11) that leads to the formation of the SS18-SSX fusion gene. The follicular dendritic cell sarcoma expresses S-100 protein and epithelial membrane antigen, as well as CD21 (Epstein-Barr virus receptor on B lymphocytes), CD23 (receptor FcεRII for IgE), and CD35 (a monocyte marker). Neural tumors are particularly difficult to differentiate from MMM. However, tumors of neural crest origin, such as neurothekeomas and schwannomas, are S-100 positive but usually HMB45 negative.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








