With medical advancements and an increased life expectancy in modern society there has been heightened interest in optimizing one’s quality of life. More than ever, there exists the desire to undo or reverse the facial skin changes that result from sunlight, aging, and genetics. A myriad of technological and medical “breakthroughs” have been produced to meet this demand, yet many are unsubstantiated. Chemical peels remain one of the steadfast and proven tools for facial skin resurfacing. Within this chapter, the advances and techniques associated with the multilevel phenol–croton oil peels are highlighted.
Chemical peels are primarily performed for treatment of rhytids, lentigines, dyschromias, and actinic skin damage. Numerous chemical-peel formulations have been described. Although the art of chemical peeling, or chemexfoliation, has been practiced for centuries, modern-day use was initiated in Hollywood, California, in the 1920s by lay peelers. In the 1950s and 1960s, use of chemical peels moved to the hands of physicians and there was a movement to standardize its practice. Out of this was born the classic Baker-Gordon phenol–croton oil formulation that served as the “gold standard” for deep chemical peels.1 This peel was utilized by practitioners for decades, yet carried with it the risk of potentially significant complications due to its aggressive nature. In 2000, Hetter eloquently described his findings associated with the phenol–croton oil peel and helped to dispel much of the previously accepted dogma. The publications of Hetter and later Stone serve as the foundation for the advancements that have subsequently occurred in the use of phenol–croton oil peels.
25.2 Patient Selection
Defining the appropriate patient for a chemical peel is of utmost importance. This requires careful attention to both the patient’s physical and mental attributes. Physically, one must adhere to appropriate indications, namely rhytids and skin photodamage in the forms of dyschromias and actinic damage (▶ Fig. 25.1). These features must be distinguished from gravitational and other senescent changes including jowling and facial lipoatrophy. Patients who are to undergo phenol–croton oil peels are ideally characterized as those with fair skin, blue eyes, and shallow rhytids. The Fitzpatrick skin type classification is frequently utilized to categorize appropriate patients (▶ Table 5.1).2 In general, patients with Fitzpatrick skin types I, II, and sometimes III are appropriate patients for the phenol–croton oil peels. Another categorization scheme that is helpful in rating patient skin quality and severity of photodamage is the Glogau skin classification (▶ Table 25.1).3

Fig. 25.1 Preoperative photograph of patient demonstrates fair skin, diffuse fine rhytids, and scattered lentigines.
Classification type | Age group | Wrinkling | Typical characteristics |
I―Mild | 20–30s | No wrinkles | Mild pigmentary changes, minimal wrinkling, no keratoses, little makeup required |
II―Moderate | Late 30s or 40s | Wrinkles in motion | Early lentigines, palpable but not visible keratoses, parallel smile lines appear, foundation makeup usually necessary |
III―Advanced | 50s | Wrinkles at rest | Obvious dyschromias, telangiectasias and keratoses, heavy foundation makeup required |
IV―Severe | 60–70s | Only wrinkles | Severe photoaging, yellow-gray skin color, history of cutaneous malignancies, no normal skin, cannot wear makeup (cakes and cracks) |
Modified with permission from Glogau RG. Aesthetic and anatomic analysis of the aging skin. Semin Cutan Med Surg 1996; 15: 134–138. | |||
During the initial patient consultation one must develop a sense of the patient’s mental preparedness and expectations for a chemical peel. Optimal patient outcomes often necessitate strict adherence to preoperative and postoperative regimens. Lifestyle activities need to be assessed. Patients with habitual sun and smoke exposure should be counseled on the damaging effects that ultraviolet (UV) radiation and smoke can cause in the first several months following a peel. Those patients who cannot abide by these limitations should be recommended for alternative therapies.
Realistic expectations should be delineated according to the depth of the phenol–croton oil peel to be performed. Erythema is an expected temporary consequence that may last up to 3 months. Patients should ideally be comfortable with the idea of wearing concealing makeup in the intervening time period. To provide the patient with an approximate estimate of the final skin quality following the chemical peel, one may use the patient’s axillary skin―provided there has been no history of significant UV exposure. Standardized preoperative photographs should be taken to document the degree of photodamage.
Patients should be screened for other contraindications to performing resurfacing procedures. Relative contraindications include active or frequently recrudescent oral herpes simplex virus (HSV) infections, history of head and neck radiation, diabetes, hypertrophic or keloid scars, and connective tissue disorders. Any patient with elevated estrogen levels is a poor candidate for chemical peels due to the risk of hyperpigmentation. Therefore, those taking oral contraceptives, exogenous hormone replacement, or those planning to become pregnant within 6 months following a peel should be cautioned. Photosensitizing drugs also need to be avoided.
One’s ability to re-epithelialize following a chemical peel is dependent upon adnexal structures within the dermis. Any activity that inhibits this process should be considered a contraindication to performing a chemical peel. Isotretinoin use within 6 to 12 months is regarded to be an absolute contraindication due to its inhibition of sebaceous gland activity.
25.3 Choosing Depth of Peel
After selecting appropriately indicated patients, the correct peel depth must be determined. The depth of the chemical peel is based upon the level of skin penetration. Superficial peels are designated as those that induce tissue necrosis limited to the epidermis and will not be included in the discussion of modified phenol–croton oil peels. Medium-depth peels result in destruction of the epidermis with variable necrosis and inflammation of the papillary dermis. Inflammation may extend to the superficial reticular dermis. Deep peels result in epidermis and papillary dermis tissue destruction with inflammation extending to the midreticular dermis. Any deeper tissue destruction would potentially damage the adnexal structures and raise the risk of dyspigmentation and scarring.
Medium-depth chemical peels are often utilized for patients with Glogau level II photodamage. The medium-depth peel can also improve mild and moderate acne scarring. Patients with Glogau levels III and IV are often best treated with deep chemical peels. Specific areas of the face may be treated with different peel depths. Deep rhytids in areas where the skin is thicker, such as the glabella and perioral regions, are better addressed with deep peels, whereas the nasal dorsum and periorbital areas are better suited for medium-depth peels. The Fitzpatrick skin type of a patient should also be a consideration. Greater depth of skin penetration raises the risk of postinflammatory hyperpigmentation (PIH), hypopigmentation, and scarring; therefore, deep peels should be reserved for Fitzpatrick skin types I and II.
25.4 Preprocedural Preparation
A number of prepeel measures may help to optimize healing and final skin outcomes. UV protection in the form of broad-spectrum ultraviolet A (UVA) and ultraviolet B (UVB) sunscreen with at least sun protection factor (SPF) 30 is recommended up to 3 months prior to performing the peel. Doing so stabilizes melanocyte activity and helps prevent prepeel burns or tanning. Sunlight exposure should also be minimized during this time.
Tretinoin is a topical therapy that may be initiated 6 weeks before the peel. There are a number of benefits that can result. Tretinoin aids in re-epithelialization and induces increased melanin distribution.4 Following tretinoin therapy, the epidermis is thickened and demonstrates decreased corneocyte adhesion, reduced stratum corneum thickness, and neocollagen production. The thickened and uniform epidermis optimizes the execution of the peel, which correlates with improved final results.5 The recommended dose range is between 0.025 and 0.1% and application is nightly. Possible side effects include erythema, skin irritation, and flaking. For patients sensitive to the effects of tretinoin, treatment should be started with lower concentrations.
Topical hydroquinone (4–8%) and hydrocortisone (1–2.5%) are also effective prepeel medications and can be started 6 weeks prior to the peel. Hydroquinone is particularly effective for patients with lentigines, dyschromias, and patients with higher Fitzpatrick skin types (e.g., types III and IV) because there is an increased risk of PIH in these patients. Hydroquinone inhibits the enzyme tyrosinase within melanocytes, which blocks melanin production. The application of a topical steroid such as hydrocortisone will help to minimize any inflammatory processes prior to undergoing the peel. In treatment of pigmentation, meta-analysis has demonstrated superior improvement with triple combination therapy (tretinoin, hydroquinone, topical steroid) than with monotherapy alone.6
All patients undergoing medium and deep peels should be started on antiherpetic prophylaxis. Even if a patient has no prior history of oral HSV outbreaks, the individual should be counseled about that possibility. Latent infections may arise even in the setting of a negative history. Patients with a negative history are regularly started on acyclovir 400 mg three times daily, starting 3 days prior to the peel and continuing 10 to 14 days following the procedure until re-epithelialization is complete. Anyone with a positive history for oral herpetic outbreaks should be provided 1 g of valacyclovir three times daily for the same duration. Herpetic outbreaks following a chemical peel can be devastating to the final results and all precautions should be undertaken to prevent their occurrence.
Because chemical peels intentionally remove the epidermis and portions of the dermis, there is potential for bacterial contamination. Cutaneous flora such as staphylococcal and streptococcal species are the typical causative agents in postpeel cellulitis. Because of its additional utility in treating acne, the authors initiate prophylactic minocycline, 100 mg once daily, starting the day prior to the peel and continuing for 10 days total. Acceptable alternatives include clindamycin, 150 mg three times daily; cephalexin, 250 mg four times daily; or erythromycin, 250 mg four times daily, for the aforementioned duration.
Other precautions should be discussed with the patient to improve uniform depth of penetration during the peel and overall healing. These include avoidance of facial skin waxing, dermabrasion, and electrolysis for 2 to 3 weeks prior to the peel. The chemical peel may be combined with other facial procedures; however, the authors caution against combining the procedure with rhytidectomy procedures as well as transcutaneous lower eyelid blepharoplasty. Decreased blood flow from skin-flap elevation during rhytidectomy may compromise blood flow and impair healing. It is recommended to stagger the peel and rhytidectomy by 6 weeks. Transcutaneous lower eyelid blepharoplasty, combined with the skin-tightening effects of a periorbital chemical peel, places the patient at undue risk for postoperative ectropion.
25.5 Multilevel Phenol–Croton Oil Peels
The multilevel phenol–croton oil peels represent an evolution within medium and deep chemical peels. Aside from the Baker-Gordon phenol-based peel, the only other agent capable of attaining the similar depths was 50% trichloroacetic acid (TCA). Unfortunately, 50% TCA has been shown to significantly increase the risk of postoperative scarring, despite attempts to optimize conditions with various surfactants, emulsifiers, or other additives.7,8 To achieve depths similar to the 50% TCA peel, but with a more acceptable side-effect profile, multiple combination therapies were developed. Medium-depth peels were described involving application of 35% TCA combined with other agents such as solid carbon dioxide (CO2), Jessner’s Solution, or glycolic acid.9,10,11 Brody and Hailey9 and later Brody alone12 found these three combination peels to have a scarring risk of less than 1%.
Use of the TCA-based peels flourished as a result of the all-or-none qualities of the Baker-Gordon deep peel. For years, there were unsubstantiated assertions made about the nature of the phenol–croton oil peel. These claims were accepted as dogma as early as the 1960s, when this peel was introduced into the plastic surgery arena. The original phenol–croton oil peels were in use by lay peelers in Hollywood, CA, in the 1920s. From this source, as well as Miami, Florida, in the 1950s, physicians were able to extract the lay formulas.13 Interestingly, most peels contained similar concentrations of croton oil. Litton was the first practitioner to present a phenol–croton oil formula to the American Society of Plastic and Reconstructive Surgery in the late 1950s. However, Baker received much attention for his version, which was presented in November 1961 and eventually modified to the classic formulation in 1962 (▶ Table 25.2).14
Ingredient | Volume, % total |
Phenol USP 88% | 3 mL, 49 |
Distilled water | 2 mL, 44 |
Septisola | 8 guttas, 4.5 |
Croton oil | 3 guttas, 2.1 |
Abbreviation: USP, United States Pharmacopeia. Note: 27 guttas = 1 mL. aSandent Co., Murfreesboro, Tennessee. Modified with permission from Baker TJ. Chemical face peeling and rhytidectomy. A combined approach for facial rejuvenation. Plast Reconstr Surg Transplant Bull 1962; 29: 199–207. | |
Intimately linked to the early dogmas of the phenol–croton oil peel were the descriptions presented by Adolph Brown et al.15 Three primary assertions were outlined: first, that increased concentrations of phenol prevented deeper peels by inducing superficial keratocoagulation that inhibited further penetration deeper into the dermis. Second, Brown claimed that the addition of a saponin lowered surface tension and facilitated augmented depth of penetration by phenol. Finally, he said that croton oil must be included in the solution to act as a “buffer.” The literature of the 1960s adopted these premises and then went on to declare further assertions, including that phenol was the only active ingredient within the Baker-Gordon formula and that there was an inverse relationship between the concentration of phenol and the depth of skin penetration. From the time of Brown’s initial determinations in the 1960s, there were no well-designed animal or human studies supporting these notions until the 1990s, when Gregory Hetter questioned the role of each of the components in the phenol–croton oil formula.
To begin, a description of croton oil is necessary.16 This compound is isolated from the seeds of Croton tiglium, a small shrub found naturally in India and Sri Lanka. Croton oil is a combination mainly of oleic, linoleic, myristic, and arachidonic acids. Less than 5% of the oil is composed of a resin—this resin has been known for more than a century in the scientific literature to be a skin irritant and possess toxic properties. In 1935, Joseph Spies detailed the toxic properties of the resin as he applied it to the arms of volunteers and observed severe vesiculations of the skin requiring up to 3 weeks to heal.16 He also noted the resin’s poor solubility in a 50:50 phenol to water solution. Hetter elucidated that the real reason to include Septisol (Sandent Co., Murfreesboro, Tennessee), a known surfactant, in the Baker-Gordon formula was to increase the components’ solubility.
In Hetter’s attempt to disprove the original notions regarding the phenol–croton oil peel, he performed a series of peels at different croton-oil concentrations upon one patient who agreed to undergo the treatments.16 His findings are the basis for the multilevel phenol–croton oil peels in use today. At the lowest concentration of 18% phenol there was minimal effect. Mild epidermal keratolysis subsequently occurred using 35% phenol, but there was no clear dermal effect. Mild dermal involvement was noted at 50% phenol application. Finally, using 88% phenol, Hetter observed an obvious dermal effect that required 4 to 5 days to heal. The authors use this concentration of phenol alone to achieve a very superficial medium-depth peel.
After discovering the effects of phenol alone, different concentrations of croton oil were added to the peel solution. A croton-oil concentration of 0.7% required 7 days to heal, whereas 2.1% croton oil, equivalent to the concentration in the Baker-Gordon formula, required 11 days to heal.16
Based on his observations from these series of treatments, Hetter challenged the previous dogmas regarding phenol–croton oil peels. He deduced that there was a direct correlation between phenol concentration and depth of skin penetration. Furthermore, he showed that the depth of penetration is further enhanced with the addition of increasing concentrations of croton oil. These assertions are consistent with prior investigations by Stegman17 and subsequent animal model studies.18
After completing his treatments on this patient, he used his new-found suppositions in treatment of five additional patients.19 From these patients he was able to make additional assertions regarding the phenol–croton oil peel. First, he noted that the dilution of croton oil in a constant concentration of phenol shortened patient healing time, suggesting a more shallow depth of penetration. Next, he generalized that phenol concentration has minimal impact on overall depth of tissue injury. He also observed that multiple coats of the peel agent increase the depth of injury, which was later supported in the work of Stone and Lefer.20
Johnson et al first described the need for different depths of peeling for individual subunits of the face (▶ Fig. 25.2).21 Hetter applied this concept by altering the croton-oil concentration in different regions of the face. He found that the nose could tolerate croton-oil concentrations up to 1.2%; however, the cheeks and forehead were limited to 0.8% concentrations and the upper nose, temple, and lateral brow could only tolerate concentrations up to 0.4% croton oil. Overall, Hetter felt that 1% croton oil was the upper threshold before placing the patient at increased risk of scarring and hypopigmentation.19
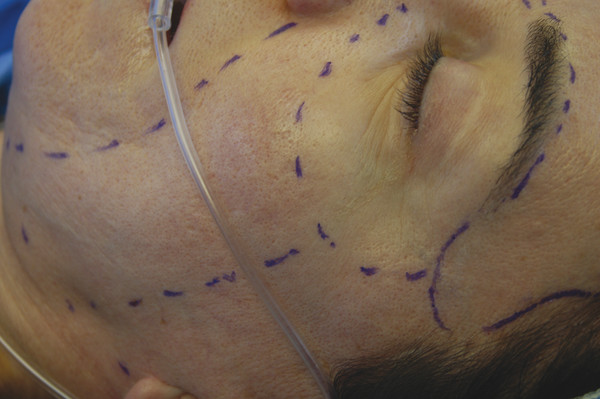
Fig. 25.2 Delineation of facial subunits to customize levels of treatment.









