Chapter 45 Molecular Methods to Prevent Adhesion Formation
This chapter does not appear in the print edition.
Outline
Clinical Significance
From clinical experience, it can be seen that three factors (“quality of repair,” “motion” in the sense of early exercise, and “inflammation”)1 have an impact on the degree of adhesion formation. While the quality of a tendon repair is still up to the surgeon, strategies from the toolbox of molecular medicine are under investigation to modulate tendon healing on the molecular or cellular levels, to decrease those inflammatory effects that are responsible for adhesion formation, and to find new ways to prevent loss of tendon gliding.
Pathophysiology
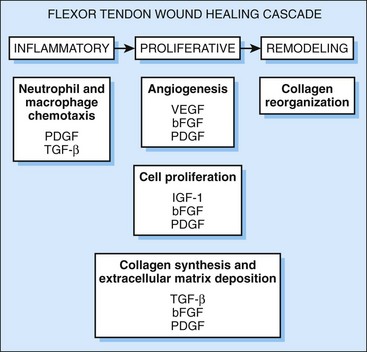
In its healing process, the tendon undergoes three distinct phases: inflammation, proliferation, and maturation/remodeling. The inflammatory phase is characterized by the migration of T-lymphocytes and phagocytotic cells into synovial sheath and epitenon. Platelets adherent at the injury site release a variety of cytokines such as platelet-derived growth factor (PDGF), transforming growth factor (TGF)-β, or insulin-like growth factor (IGF) that activate the migrating cells.2 These migrating cells are responsible for phagocytosis of cell debris and for deposition of a preliminary extracellular matrix.
In the proliferation phase, collagen synthesis and vascularization are enhanced.3 The remodeling phase is characterized by rearrangement and organization of collagen fibers.4 At this state, adhesions become more apparent.
In contrast to many other tissues, there are two different mechanisms involved in tendon healing: the extrinsic and the intrinsic pathway. In extrinsic healing, extracellular matrix producing cells are invading the lacerated tendon from its periphery. This pathway described by Potenza and colleagues5 was for some time considered to be the prevailing and maybe only mechanism of healing in a lacerated tendon.
Lundborg and others, however, have shown that tendon healing is also possible independent from the adjacent tissue. When a sutured tendon was kept inside the knee joint space of a rabbit without contact to a tendon sheath or other connective tissue surfaces, the tendon healed without forming adhesions. This intrinsic healing process allows a restitution of tendon continuity by cells out of the epitenon and endotenon without forming adhesions to its surrounding.6 McGrouther and colleagues showed in vitro that the extrinsic pathway naturally is the more active one and starts to be active at an earlier time-point.7 This means that tendon healing naturally tends toward forming adhesions and that a therapeutic approach should aim at enhancing the intrinsic pathway and suppressing the extrinsic.
Treatment
Conventional Pharmacological Treatment
Before the era of molecular medicine, various pharmacological agents have been investigated. Local and systemic steroids, nonsteroidal anti-inflammatory drugs, hyaluronic acid, 5-fluorouracil, and others have been used. However, none of these substances have been suitable for routine clinical use. Lubricin, a mucinous glycoprotein responsible for the boundary lubrication of articular cartilage, has been used to prevent adhesion formation in a canine model of tendon repair.8 The application of this substance led to a decreased adhesion formation, although it is at the expense of repair strength. Clinical value of this agent therefore is yet to be elucidated.
Biomaterials to Prevent Adhesion Formation
As described earlier, the majority of adhesion formation is considered to be caused by extrinsic rather than by intrinsic healing. Therefore, attempts with blocking materials and membranes have been made to prevent adhesion formation caused by the exterior. Before the area of molecular treatment, attempts have been made to use materials such as silicone or gelatin sponge. Using hydroxyapatite or alumina sheaths for covering a tendon suture site in the chicken model,9 Siddiqi and colleagues showed a reduction of adhesions at the plantar site, but mild to moderate adhesions both at the ends of the sheaths and at the plantar contact site to the bone. Ishiyama and colleagues reported a phospholipid polymer hydrogel to effectively prevent adhesion formation in a rat and in a chicken model.10 They explained this by a microstructure with a pore size less than 8 to 10 µm that is capable of preventing extrinsic inflammatory cells from getting into contact with the tendon surface but allowing the passage of cytokines and growth factors required for healing. Tenenhaus and colleagues11 claimed a similar mechanism to be active in adhesion prevention when they wrapped tendon repair sites with a collagen-GAG membrane (Integra) in a chicken model. This material has been used particularly in burn surgery and is known to possess good biocompatibility.
Motion
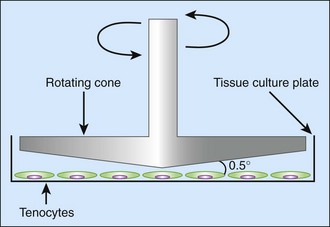
From clinical experience, it is known that early active motion treatment helps to prevent adhesion formation after tendon repair. Various explanations for this observation can be conceived. First, it might be due to mechanical reasons that newly forming adhesions are interrupted by motion. Recent research, however, has also shown an effect of motion on a molecular level. Strain and shear forces can be exerted onto cultured cells by using a cell bioreactor, which is simulating the conditions of motion.12 By exercising mechanical shear stress on tenocytes in vitro (Figure 45-1), Fong and colleagues showed an “antifibrotic” gene expression pattern—that is, an upregulation of matrix-metalloproteinases and a downregulation of collagen I and III as well as of TGF-β.13 Not only cells in culture but also cell-seeded grafts made from acellularized cadaver tendons can be treated by mechanical force before implantation. Angelidis and colleagues used a cell bioreactor to exert a cyclic load of 1.25N on cell-seeded tendon scaffolds for 5 days. Subsequent biomechanical testing showed higher tensile strength of the bioreactor-treated tendons compared to untreated controls. Moreover, the seeded cells (adipoderived stem cells and skin fibroblasts) showed an orientation parallel to the direction of force on the scaffold. Further studies will have to show whether this orientation also helps to prevent adhesion with the surroundings.
Prevention of Adhesions in Tendon Grafting
The formation of adhesions is an even greater problem in tendon grafting than it is in tendon repair. Commonly used grafts such as the palmaris longus tendon lack an extrasynovial sheath and are therefore highly susceptible to adhesion formation. Furthermore, the function of artificial grafting materials has been poor. On the other hand, the use of allografts or xenografts is limited due to immunological reasons, as these antigenic stimuli generally lead to excessive fibrosis, a fact that is most detrimental in the gliding region of zone 2.14 Acellularization of cadaver tendons could be a way to obtain a nonimmunogenic scaffold for tendon repair with the right mechanical properties. Zhang and colleagues acellularized rabbit tendons by treating them with trypsine/EDTA + Triton-X solution.15 These acellularized scaffolds could be repopulated with epitenon and endotenon cells. Reseeding the acellularized tendons with tenocytes has been shown to improve their biomechanical properties, mainly ultimate tensile strength and elastic modulus.16 It may be speculated that the newly formed epitenon layer (Figure 45-2) can also inhibit adhesion formation, which has to be proved in further preclinical studies.
Cytokines
As a variety of cytokines are active in the process of tendon healing (Figure 45-3), blocking the “proadhesion” and substituting the “antiadhesion” ones could be a powerful strategy in preventing tendon adhesions. However, there are several obstacles to overcome before this approach will come to fruition. The number of possible cytokines involved is large, and there may be several cytokines that are still unidentified. Second, the above classification into “proadhesion” and “antiadhesion” is overly simplified. A cytokine may perform contradictory effects depending on the time and location of its activity. The exact regulation of cytokine secretion and activation is regulated by various paracrine feedback loops that will be very difficult to simulate. Nevertheless, promising results already have been obtained when certain cytokines were either substituted or blocked.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree